INTRODUCTION
Chlamydia pneumoniae is a common respiratory pathogen and has a tendency to give rise to chronic infections. The association between C. pneumoniae infections and coronary heart disease (CHD) has been documented in several studies [Reference Saikku1, Reference Leinonen and Saikku2]. CHD is a multifactorial disease, and number of risk factors, such as hypertension, hyperlipidaemia, smoking and obesity, have been identified. Recently, the association between C. pneumoniae and the risk factors of CHD have been studied intensively.
In obesity, adipose tissue is inflamed and many inflammatory molecules, such as interleukin 6 (IL-6) and tumour necrosis factor-α (TNF-α), are produced [Reference Hotamisligil, Shargill and Spiegelman3, Reference Mohamed-Ali4]. This low-grade inflammation in adipose tissue induces insulin resistance and obesity, which are linked to metabolic syndrome, type 2 diabetes and CHD [Reference Xu5–Reference Ross7]. In addition to genetic and environmental factors, infections have also been associated with obesity. It is now known that infections and microorganisms can cause adipocyte dysfunction and inflammation. This association was first demonstrated between adenovirus and obesity in humans [Reference Dhurandhar8, Reference Dhurandhar9]. Recently, many other microorganisms and also C. pneumoniae have been linked to an elevated body mass index (BMI) and other CHD risk factors [Reference Ekesbo10–Reference Dart, Martin and Kay12].
Slightly elevated serum C-reactive protein (CRP) levels act as a marker of systemic inflammation, and have been shown to increase the risk of CHD [Reference Danesh13]. The synthesis of CRP is regulated by IL-6, which has been assumed to originate largely from adipose tissue. Thus, CRP has been associated with insulin resistance and obesity, as well [Reference Visser14, Reference Yudkin15].
In the current study we investigated whether elevated C. pneumoniae IgG antibodies and slightly elevated CRP levels were associated with an increased BMI in young Finnish military recruits. We were especially interested in persistently elevated antibodies, a possible indicator of chronic infection, during military service.
METHODS
Study subjects and specimens
The study population included 891 military recruits (228 with and 663 without asthma) from the July 2004 and January 2005 intake groups for military service in Kajaani Garrison, Kainuu Brigade, in northern Finland. All the men who agreed to participate in the study signed an informed consent form. More than half (58·2%) of the participants served for 6 months, 6·2% for 9 months, and 27·6% for 12 months, and the study was aborted by 8·1%. The age of the participants ranged from 17·4 to 29·6 years (mean 19·6 years). The study participants were Finnish men all of whom were of white European ancestry. The study protocol was approved by the Medical Ethics Committee of the Kainuu Central Hospital.
Asthma
Asthma was defined as previously diagnosed by a physician according to data from previous health and call-up examinations.
Body mass index (BMI)
The body height and weight of the conscripts were measured during health examinations at the beginning of their military service. BMI was calculated as weight in kilograms divided by the square of height in meters.
Smoking
Smoking was classified as smokers and non-smokers, based on a questionnaire at the beginning of the military service. Smokers were current daily smokers. Non-smokers had never smoked or had stopped smoking or smoked only occasionally.
Education
Information on education was collected from the conscripts' personal data forms and classified as comprehensive school, or vocational school, or upper secondary school education.
Laboratory methods
Sera were obtained at the beginning and end of the participants' military service. The samples were stored at −80°C until analysed.
Microimmunofluorescence (MIF) test
A MIF test was used to measure C. pneumoniae IgG antibody levels in the serum samples [Reference Wang16, Reference Paldanius17]. Using Finnish C. pneumoniae strain Kajaani 6 (K6) elementary bodies as an antigen, serial fourfold dilutions of the serum samples, starting from 1:32, were prepared for detection of IgG antibodies. Positive serum controls with known titres were included in each series. The seropositivity cut-off value for IgG was ⩾32. The presence of IgG antibodies in arrival and departure serum samples during 180, 270, or 362 days' military service was considered as persistence of antibodies and a possible indication of chronic C. pneumoniae infection. A ⩾fourfold rise in IgG antibodies between the arrival and departure samples was considered a seroconversion and a possible indication of clinical or subclinical C. pneumoniae infection. Arrival serum samples were available from 888 conscripts and both arrival and departure sera, from 794 conscripts.
Highly sensitive C-reactive protein (hsCRP)
The hsCRP levels were determined by the immunoenzymometric assay (IEMA) test (Medix Biochemica, Finland) ([Reference Taponen18], and according to the manufacturer's instructions) from the blood samples. The assay range was from 0·3 to 30 mg/l, and the sensitivity of the test was 0·08 mg/l. The lowest hsCRP level of the highest quartile was used as a cut-off value for elevated hsCRP levels. To limit the possible increasing influence of infections on CRP level, only conscripts with CRP <10 mg/l were included in the CRP analysis (n=809). A persistently elevated CRP level was defined as a CRP level above the upper quartile in the arrival and departure samples during military service.
Statistical analysis
To test the significance between BMI groups, an independent-samples t test was used for continuous variables, and Pearson's χ2 test and χ2 linear-by-linear association test were used for categorized variables. For BMI and hsCRP that did not follow normal distribution, a logarithmic transformation was done. For multivariate analyses, odds ratios with a 95% confidence interval (CI) were estimated by logistic regression, and analysis of variance was used to obtain geometric mean BMI values in different groups. Statistical analyses were performed using SPSS version 15.0 (SPSS Inc., USA).
RESULTS
The geometric mean BMI in the study population of 891 military recruits at arrival for military service was 24·2 kg/m2 (95% CI 23·9–24·4 kg/m2) and the range was from 16·0 to 42·9 kg/m2. The study population was divided into two groups according to BMI; normal weight (BMI <25 kg/m2, n=552) and overweight (BMI ⩾25 kg/m2, n=339) conscripts. There were 92 obese conscripts with a BMI ⩾30, who were included in the overweight group. The distribution of different variables in these groups is shown in Table 1. Overweight conscripts were slightly older than normal weight conscripts (P=0·036). There was no statistically significant association for asthma or smoking between the BMI groups (P=0·501 and P=0·179, respectively). Education was associated with BMI; the conscripts with comprehensive school or vocational education were more often overweight than were those with upper secondary school education (P<0·001). The prevalence of IgG seropositivity (⩾32) in 888 military recruits at arrival was 46%, and seropositivity was significantly more prevalent in overweight than in normal weight conscripts (54% vs. 41%, P<0·001). The prevalences of persistent IgG and IgG seroconversion in 794 conscripts were 30% and 13%, respectively. Persistent IgG antibodies were significantly associated with overweight (P<0·001), whereas IgG seroconversion was not (P=0·440) (Table 1). The geometric mean hsCRP concentration in 809 military recruits at arrival was 0·84 mg/l. The difference in the mean CRP concentration between normal weight and overweight conscripts was significant (0·69 mg/l vs. 1·16 mg/l, P<0·001). The upper quartile of CRP concentration in the arrival and departure samples was 1·70 mg/l. A persistently slightly elevated CRP level (defined as a CRP level ⩾the upper quartile on arrival and departure) was associated with the overweight group (P=0·007) (Table 1). In addition, a positive correlation between BMI and hsCRP at arrival (r=0·234, P<0·001) and C. pneumoniae IgG titre (r=0·130, P<0·001) was observed (data not shown).
Table 1. Descriptive statistics of the study population
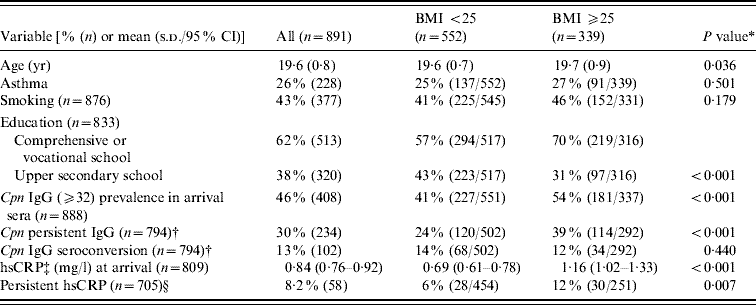
BMI, Body mass index; CI, confidence interval; Cpn, C. pneumoniae; CRP, C-reactive protein; s.d., standard deviation.
* Independent-samples t test for continuous variables, Pearson χ2 for categorized variables.
† IgG antibody persistence and seroconversion in arrival and departure sera.
‡ Geometric mean.
§ hsCRP level ⩾upper quartile (1·7 mg/l) on arrival and departure.
In the logistic regression analysis, an elevated CRP level at arrival (OR 2·0, 95% CI 1·4–2·7), a persistently high CRP level (OR 2·2, 95% CI 1·3–3·9), C. pneumoniae IgG seropositivity (OR 1·5, 95% CI 1·1–2·0), and persistent IgG antibodies (OR 2·1, 95% CI 1·5–2·8) proved to be significant risk factors of overweight when adjusted for intake group and education (Table 2). To further analyse the significance of persistent infection and inflammation, those who had a persistently elevated CRP level and persistent antibodies during military service, and those who had either of them, were compared with those who had neither of them. A significant risk was observed in these groups (adjusted OR 3·0, 95% CI 1·2–7·9 and adjusted OR 2·2, 95% CI 1·6–3·2, respectively) (Table 2). Adjusting the analyses for asthma status and smoking did not alter the results. In addition, statistically significant trends were found between normal weight, overweight, and obese groups; P<0·001 for elevated CRP level, P=0·001 for persistent CRP level, P=0·009 for IgG seropositivity, P<0·001 for persistent IgG and P<0·001 for combined persistent CRP and persistent antibodies (data not shown).
Table 2. Adjusted odds ratios for overweight (BMI ⩾25) and adjusted geometric mean values of BMI in different groups of hsCRP level and C. pneumoniae IgG antibodies
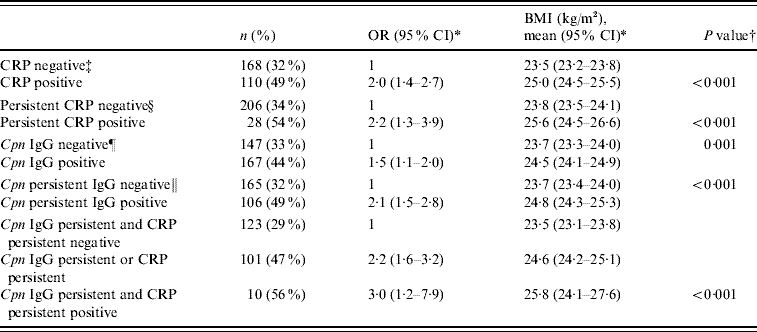
BMI, Body mass index; CI, confidence interval; Cpn, C. pneumoniae; CRP, C-reactive protein; OR, odds ratio.
* Multivariate analyses were adjusted for intake group and education.
† P value for the difference between geometric mean values of BMI.
‡ hsCRP level ⩾upper quartile (1·7 mg/l) on arrival.
§ hsCRP level ⩾upper quartile on arrival and departure (1·7 mg/l).
¶ IgG titre ⩾32 on arrival.
∥ IgG positive in arrival and departure sera.
BMI values together with covariances were analysed in the different groups. Asthmatic status, smoking, intake group, and education were included in the model. Asthma status and smoking were not statistically significant and were excluded from the final model. Table 2 shows the geometric mean BMI values for categorized hsCRP concentration, persistent CRP, C. pneumoniae IgG seropositivity, persistent antibodies, and combined persistent CRP and persistent antibodies. Those who had a persistently elevated CRP level and antibodies, or those who had either of them, had a significantly higher BMI compared to those who had neither of them (25·8 kg/m2vs. 24·6 kg/m2vs. 23·5 kg/m2, respectively; P<0·001) (Table 2).
DISCUSSION
In the current study, C. pneumoniae IgG antibodies and slightly elevated CRP levels were significantly associated with overweight. In particular, an association was detected for persistence of antibodies and elevated CRP concentration and, furthermore, for the combined presence of persistent IgG antibodies and persistently high CRP. The presence of IgG antibodies in any serum can be considered a mark of previous exposure to C. pneumoniae and when present persistently may suggest prolonged or chronic infection. Interestingly, a possible acute C. pneumoniae infection, diagnosed by antibody seroconversion during the 6–12 months of service, was not associated with BMI at all. This finding suggests that possible persistent C. pneumoniae infection, but not an acute infection, predisposes to overweight. On the other hand, overweight may induce the chronicity of C. pneumoniae infection. The causality can only be speculated upon here.
Previously, the association between C. pneumoniae and obesity has mainly been shown in studies where single-point antibody measurements have been used to indicate earlier exposure to infection. First, Ekesbo et al. [Reference Ekesbo10] found that combined seropositivity for Helicobacter pylori and C. pneumoniae or either of them was associated with a high BMI and high fasting levels of insulin. Dart et al. [Reference Dart, Martin and Kay12] also reported an association between C. pneumoniae seropositivity and cardiovascular risk factors, especially increased body weight. Karppinen et al. [Reference Karppinen11] found that possible chronic C. pneumoniae infection, defined as persistent IgG or IgA antibodies in two serum samples taken at least 1 month apart, was associated with increased BMI levels in patients with sciatica. In our previous study [Reference Lajunen19], elevated serum chlamydial lipopolysaccharide (cLPS) and C. pneumoniae IgG seropositivity, suggesting active frequent or chronic infection, was associated with an elevated BMI in patients with cardiovascular disease. In a recent study by Thjodleifsson et al. [Reference Thjodleifsson20] elevated BMI levels were more frequent in those with elevated IgG antibody level for C. pneumoniae, as well as for H. pylori. On the contrary, neither Kaftan & Kaftan [Reference Kaftan and Kaftan21] nor Koziolek et al. [Reference Koziolek22] found any association between BMI and C. pneumoniae IgG seropositivity. The interaction between C. pneumoniae and changes in lipid metabolism has also been studied in our group earlier [Reference Leinonen and Saikku23, Reference Leinonen24]. In Finnish reindeer herders, persistent C. pneumoniae antibodies over a period of 3 years were associated with lower HDL cholesterol and higher triglycerides [Reference Laurila25] and, in addition, with obesity (BMI ⩾28 kg/m2) [Reference Leinonen and Saikku23].
Besides C. pneumoniae and H. pylori, several viruses in animals and two viruses, SMAM-1 avian adenovirus and human adenovirus Ad-36, in humans have been associated with obesity [Reference Dhurandhar8, Reference Dhurandhar9, Reference Atkinson26]. Ad-36 is able to infect adipocytes and recently, it was also shown that C. pneumoniae is able to infect murine pre-adipocytes and adipocytes [Reference Shi27] as well as human adipocytes [Reference Bouwman28]. Ad-36 has been shown to induce adipocyte differentiation and it has been postulated that Ad-36 has a direct effect on adipogenesis and/or the accumulation of lipid by adipocytes [Reference Dhurandhar29, Reference Vangipuram30]. However, C. pneumoniae more likely promotes the secretion of inflammatory molecules, such as TNF-α, suppressing insulin signalling and adipogenesis by inflammatory mechanisms [Reference Shi27]. It has been shown earlier that C. pneumoniae is able to infect macrophages and induce lipid accumulation and foam cell formation in them [Reference Kalayoglu and Byrne31]. One factor in chronic inflammation of obesity is that macrophage cells infiltrate adipose tissue, inducing adipocyte hypertrophy [Reference Cancello and Clement32]. This may be the route that allows C. pneumoniae to get into adipose tissue. Macrophages produce the same pro-inflammatory molecules, IL-6 and TNF-α, as adipose tissue, and C. pneumoniae has been shown to induce the production of these molecules from infected macrophages [Reference Kaukoranta-Tolvanen33].
In the current study, the rate of C. pneumoniae seroconversion was similar in overweight and normal weight subject. This suggests that obese subjects are not more susceptible to acute C. pneumoniae infections. However, as the association was found for the persistent presence of elevated CRP and IgG antibodies, the association is probably connected to more generalized inflammation induced by C. pneumoniae infection in any tissues, e.g. in lungs or blood vessels and not only in adipose tissue.
Military service is mandatory in Finland and all men aged 18–19 years are called up for service. Ninety-eight percent of them attend a call-up examination to establish their fitness for military service [Reference Latvala34] and 80–85% of all men complete their service [Reference Kajosaari35]. Therefore, our study population represents well the normal healthy population of young Finnish men. The current study suggests that already at a young age infections could be a risk factor of overweight, or vice versa, whereas previously the association has been shown mainly in an older population [Reference Ekesbo10–Reference Dart, Martin and Kay12, Reference Lajunen19]. Moreover, previous studies have been mainly conducted in CHD patients, whereas our study population represents the healthy population. In our study we considered only slightly elevated CRP levels, defined as <10 mg/l, which limited the possible influence of acute infections on CRP level. In addition, serum samples taken 6–12 months apart were available and enabled the study of persistently elevated CRP concentrations as well as persistent C. pneumoniae antibodies during military service. Thus, our results strongly point to the possibility that both persistent infection and systemic inflammation are associated with obesity in these young Finnish men.
We used the MIF method, the gold standard of serological diagnosis [Reference Dowell36] to measure C. pneumoniae antibodies. It has been shown previously that inter-laboratory variation in antibody titres with subjective MIF method may be high [Reference Peeling37]. However, in the current study we only analysed the presence or persistent presence of antibodies or fourfold titre changes. Although there are no accepted serological criteria for chronic C. pneumoniae infection, it is known that even IgG antibodies do not normally persist in young persons [Reference Paldanius38]; thu, persistence of antibodies might also indicate persistence of infection. It has been suggested that the presence of short-living IgA antibodies might be a better indication of chronicity than IgG antibodies. In our study, C. pneumoniae IgA antibodies were also analysed and persistent IgA antibodies were associated with BMI (P=0·010) (data not shown). However, due to the low number of participants with IgA seropositivity in this young study population, IgA antibodies were not reported. As the study population included only men, there may be a gender bias in this study. Therefore, prospective studies in women and both genders are needed.
In conclusion, we showed here that persistent C. pneumoniae IgG antibodies as a suggestive marker of chronic infection, and slightly elevated CRP levels as a marker of systemic inflammation, were associated significantly with an elevated BMI in young adult men. Our results in a homogenous, young and healthy study population confirm previous, less controlled studies in older populations with more confounding factors. Further studies are still needed to investigate the potential mechanisms between C. pneumoniae and obesity.
ACKNOWLEDGEMENTS
We thank Seija Liikanen for technical assistance. This study was supported by the Scientific Advisory Board for Defence (MATINE) of the Ministry of Defence of Finland, project 717.
DECLARATION OF INTEREST
None.




