INTRODUCTION
Avian influenza viruses (AIVs), capable of infecting both birds and mammals, are classified in the genus Influenzavirus A in the family Orthomyxoviridae [Reference Easterday, Hinshaw, Halvorson and Calnek1, Reference Kawaoka and Fauquet2]. Antigenic subtypes are based on the amino-acid composition of the transmembrane glycoproteins, haemagglutinin (HA) and neuraminidase (NA) [3–Reference Phipps, Essen and Brown5]. There are 16 HA and 9 NA subtypes, each virus having one HA and one NA in any combination [Reference Easterday, Hinshaw, Halvorson and Calnek1, Reference Capua and Alexander6]. AIVs are maintained in wild aquatic birds, especially waterfowl, gulls, terns and waders, in which they rarely cause overt signs of disease [Reference Webster7–Reference Munster9]. The epidemiology of avian influenza is not fully understood [Reference Munster9] and, prior to completion of this study, no information was available on AIVs in waterfowl in Ireland.
In poultry, AIV infection is often associated with mild disease, usually manifested by minor respiratory signs [Reference Alexander10]. Since 1980, there have been eight detections of low pathogenic avian influenza (LPAI) viral infections in farmed birds in Ireland, seven of which were associated with clinical disease [11, Reference Campbell, De Geus and Alexander12].
AIVs of subtypes H5 and H7 may mutate to a highly pathogenic form in poultry [Reference Capua and Alexander6, 11, Reference Kawaoka13]. Highly pathogenic avian influenza (HPAI) causes direct loss to poultry production and adversely affects trade and animal welfare [Reference Capua and Alexander14, Reference Hall15]. One outbreak of HPAI in Ireland, caused by an H5N8 virus, resulted in severe disease and significant economic loss during 1983 [11, Reference Murphy, McFerran and McNulty16]. AIVs are also potentially zoonotic and their recent transmission to humans and other mammals has resulted in several fatalities [Reference Poland, Jacobson and Targonski17–20]. In 1996 in England, human infection with AIV involved a H7N7 virus similar to a turkey virus isolated in Ireland in 1995 [Reference Banks, Speidel and Alexander21]. Re-assortment between avian viruses in terrestrial and aquatic birds [Reference Li22] and between avian and mammalian viruses [Reference Ma23] creates the potential for human influenza pandemics as occurred in 1957 and 1968 [Reference Shu24]. In 1918, direct transmission to humans of an avian H1N1 virus was responsible for the largest pandemic on record [Reference Taubenberger25].
Knowledge of the occurrence, characteristics and transmission of AIVs in waterfowl in Ireland is critical to risk assessment and prevention of avian influenza in Irish poultry. Passive wild bird surveillance has provided early warning of the presence of H5N1 HPAI virus in a number of European countries [Reference Pittman26].
The Wexford Sloblands, containing about 2000 hectares of polder located on the southeast coast of Ireland, was chosen as the principal site for this study because of its unique ecological features. In winter, this site harbours thousands of migratory waterfowl from Northern Europe, Canada, Greenland and Iceland, in addition to many sedentary species [Reference Wernham27, Reference Crowe and Merne28]. Furthermore, a wildfowling syndicate releases thousands of farmed mallard onto the site every year.
The purpose of this study was to investigate AIVs in an Irish wetland site, to compare the findings from this ‘high risk’ site with results from other locations in Ireland and to use these data to assess the potential risk to domestic birds.
METHODS
Sampled populations
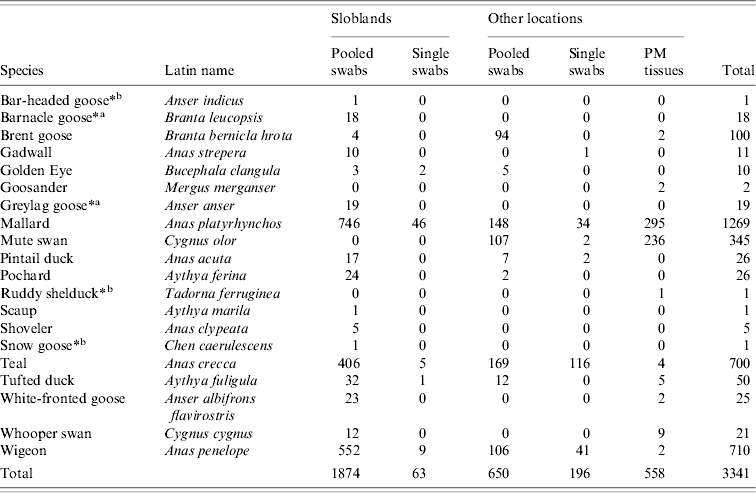
A total of 3341 waterfowl were sampled between January 2003 and September 2007. The study population comprised 1937 birds sampled at the Wexford Sloblands and 1404 birds sampled at other locations throughout the country (Tables 1, 2). Of the 3341 birds, swabs were collected from 2783, 2524 of which were sampled by cloacal swabbing only and 259 by combined cloacal and oropharyngeal (O/P) swabbing (Table 1). Specimens from 558 birds, collected during post-mortem examination, were also tested. The majority of swabs were collected from November to January to coincide with the period when the greatest numbers of migratory birds were present. During the study, birds were sampled at the Sloblands in the winters of 2002/2003 (168), 2004/2005 (607), 2005/2006 (1099) and 2006/2007 (63) (Table 2). Samples were not collected in the winter of 2003/2004. All swabs collected from ducks at the Sloblands were from apparently healthy birds that had been shot in flight. The geese and swans at the Sloblands were sampled in conjunction with other research studies while tissues were collected at post-mortem examination of birds found sick or dead. In total, specimens were collected from 12 species of duck (n=2811), six species of goose (n=164) and two species of swan (n=366) (Table 2).
Table 1. Numbers of waterfowl sampled, by pooled and individual swabs, at the Sloblands and other locations and at post-mortem (PM) examination
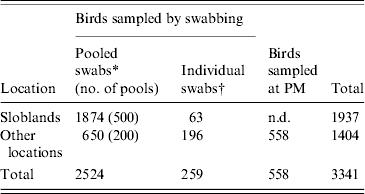
* 2002–2006: swabs pooled for testing by virus isolation in embryonated eggs; pools consisted of a maximum of five swabs collected from birds of the same species.
† 2006/2007: individual cloacal and oropharyngeal swabs from each bird tested by real-time reverse transcriptase polymerase chain reaction (rtRT–PCR).
Table 2. Waterfowl sampled in Ireland during 2003–2007 (number of each species sampled at different locations and by different methods listed)
PM, Post mortem.
* Free-living birds with pinioned wings.
a Not recent migrants. b In wildlife park.
Collection, transport and storage of specimens
Swab specimens were collected by inserting a dry sterile cotton swab into the cloaca so that it became lightly coated with faeces or into the oropharynx so that it became coated with mucus. Swabs were immediately placed into virus transport medium (VTM) consisting of Eagle's Minimum Essential Medium, with 0·25 mg/ml gentamycin, 5000 units/ml mycostatin, 10 000 units/ml benzyl penicillin and 10 mg/ml streptomycin sulphate. Cloacal swabs from the same bird species were pooled, with a maximum of five swabs/3 ml volume of VTM. Swabs collected from wild birds during the 2006/2007 shooting season were not pooled but were individually placed in 1·5 ml VTM.
Swabs collected by the project team were maintained at +4°C from sampling until delivery to the laboratory where they were stored at +4°C if testing was to be performed within 48 h, and at −70°C if testing was delayed. Before February 2006, swabs collected by hunters were placed in VTM and stored at −20°C until the end of the shooting season. During the 2006/2007 shooting season, O/P and cloacal swabs collected by hunters were delivered to local veterinary offices and stored at +4°C, pending delivery to the laboratory within 4 days.
Pooled tissue specimens (lung, liver, spleen, and kidney) and a separate intestinal specimen were collected during post-mortem examination of birds that were submitted to the Veterinary Laboratory Service.
Virus isolation (VI) and serological characterization of isolates
For all specimens collected from waterfowl (n=2592) up to the end of January 2006, VI and serological characterization were performed according to the protocol stipulated in Directive EC 92/40 [29]. In summary, 0·2 ml of material from each specimen was inoculated into five 9-day-old embryonated hens' eggs that were then incubated at 37°C. For every embryo that died during the first 24 h of incubation, another egg was inoculated to ensure that the incubation of five eggs was completed for each specimen. Thereafter, allantoic fluid (AF) was harvested from eggs as they died or at the end of 6 days' incubation and tested in the HA assay using 1% chicken erythrocytes. A second passage in eggs was performed on HA-negative AF. The HA subtype of virus isolates was determined by haemagglutination inhibition using specific antisera to each of the 16 HA subtypes, sourced from the World and EU Community Reference Laboratory, Veterinary Laboratories Agency (VLA), Addlestone, Surrey, UK. NA identification was performed by the NA inhibition test based on the standard protocol supplied by the VLA. All HA-positive AF and all AF from eggs that died after more than 24 h incubation were also tested by a rapid antigen detection assay (Rapid AIV Ag test kit; Anigen, Gyeonggi-do, Korea) to confirm the presence of AIV. All specimens from waterfowl (n=749) collected from February 2006 onwards were tested initially by real-time reverse transcriptase polymerase chain reaction (rtRT–PCR) assay, and VI was only attempted on those that gave a positive result. All isolates were submitted to the World/EU Reference Laboratory (VLA) for subtype confirmation.
RNA extraction and rtRT–PCR
An extraction method using phenol and guanidinium thiocyanate was adapted for total RNA isolation [Reference Chomczynski and Sacchi30]. Specimens (swab supernatant or tissue suspension) were lysed by the addition of 250 μl of specimen to 750 μl TRI Reagent BD (Sigma-Aldrich Ltd, Dublin, Ireland). RNA was separated using 1-bromo-3-chloro-propane and precipitated by isopropanol. The RNA was pelleted by centrifugation, washed in ethanol and dissolved in RNase-free water.
A rtRT–PCR screening assay, based on a protocol described previously [Reference Spackman31] was used to detect the matrix protein gene (M) of AIV [3]. Virus-specific RNA isolated from specimens was amplified in a one-step rtRT-PCR. Five μl of the total RNA was added to 45 μl RT–PCR reaction mixture containing 25 μl QuantiTect probe RT master mix (code 204445, Qiagen, West Sussex, UK), 0·5 μl Quantitect RT enzyme mixture, 200 nm each of forward primer M+25, reverse primer M-124, 0·06 μm of M-64 probe and made up to a total volume of 45 μl with RNase-free water. Real-time RT-PCR was performed following the protocol: 30 min at 50°C, 15 min at 95°C followed by 45 cycles of two-step PCR, 30 s at 95°C and 45 s at 58°C.
Specimens that tested positive for the M gene were retested to detect gene-specific sequences. H5 and H7 subtype-specific sequences were detected using one-step rtRT–PCR protocols modified and validated by the VLA. Five μl of the total RNA was added to 45 μl RT-PCR reaction mixture as described using 400 nm of primers H5LH1, H5RH1 and 0·06 μm H5PRO probe or LH6H7, RH4H7 and 0·06 μm H7PRO 11 probe, respectively. Real-time RT–PCR was performed with the following protocol: 30 min at 50°C, 15 min at 95°C, followed by 45 cycles of three-step PCR: 20 s at 95°C, 30 s at 54°C and 10 s at 72°C [3]. All rtRT–PCRs were performed with a Stratagene MX 3000P thermocycler (Stratagene LA, CA, USA). These test protocols and the nucleotide sequence of these primers and probes are available on VLA's website [32]. The quantities of primers and probes used during this study were adjusted to achieve optimal results with the equipment and laboratory conditions. Detection of other subtype-specific sequences was attempted using the RT–PCR protocol developed by Lee et al. [Reference Lee33].
RESULTS
Detection of AIV in swabs
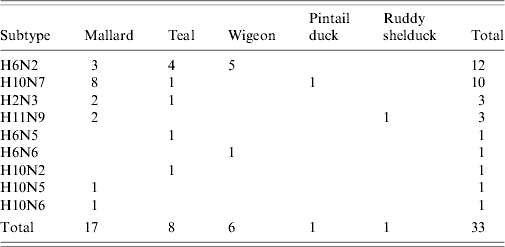
Thirty-two AIVs were isolated from pooled cloacal swabs (n=500 pools) collected at the Sloblands and tested by direct inoculation of embryonated eggs (Table 3). Nineteen isolates were obtained from birds sampled in November, eight in December and five in January, even though very similar numbers were sampled in each of these months (data not included). A single AIV isolate, an H10N5 virus, was detected in the specimens submitted during the 2002/2003 season. In the 2004/2005 season, H10N7 (n=9) was the predominant subtype isolated while in 2005/2006, H6N2 (n=12) was the most frequently isolated virus (Table 4). All of these viruses were isolated from four species of dabbling duck; mallard (Anas platyrhynchos) (n=17), teal (Anas crecca) (n=8), wigeon (Anas penelope) (n=6) and pintail (Anas acuta) (n=1) (Table 5). A strong association between subtype and duck species was not evident, although all of the isolates from wigeon were subtype H6, of which 5/6 also had the same N subtype (Table 5).
Table 3. Number of specimens positive for avian influenza by virus isolation and real-time reverse transcriptase polymerase chain reaction (rtRT–PCR)
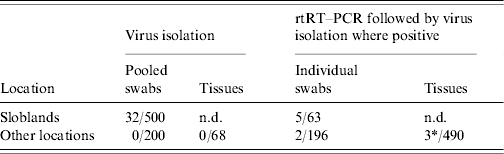
n.d., Not done.
* 1/3 rtRT–PCR-positive specimens yielded a virus isolate on passage in embryonated eggs.
Table 4. Avian influenza virus subtypes isolated from samples collected from waterfowl during 2003–2006
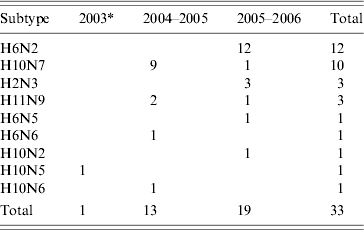
* Sampling during winter of 2002/2003 only commenced in January 2003.
Table 5. Subtypes of avian influenza virus isolated from different waterfowl species during 2003–2007
Of 259 waterfowl from which individual swabs (both cloacal and O/P) were collected during 2006/2007, seven birds (2·7%), six on cloacal and one on O/P swabs, tested positive on screening for the presence of the M gene by rtRT–PCR (Table 3). Five (1·9%) of these birds were mallard from the Sloblands and the other two (0·77%) were teal that had been sampled at other locations. Virus was not isolated from any of these M-positive specimens. However, the swabs from teal tested positive by rtRT–PCR for the H5 subtype of AIV. Sequencing of the cleavage site of the HA gene demonstrated that both of these H5 viruses were LPAI (data not included).
Detection of AIV in specimens collected from birds at post-mortem examination
Specimens collected from 68 birds at post-mortem examination were negative for AIV by VI. Tissue specimens collected from three of an additional 490 birds tested positive by rtRT–PCR for the M gene of AIV (Table 3). An H11N9 AIV was isolated from one of these specimens, the intestinal contents of a Ruddy shelduck (Tadorna ferruginea). The other two positive specimens, one from a mute swan (Cygnus olor) and the second from a mallard, were negative by VI and could not be further characterized by molecular methods.
DISCUSSION
AIVs were isolated from waterfowl sampled at the Wexford Sloblands during each winter of the study. The total of 32 AIV isolates obtained at this site over the course of the study represented a detection rate of 1·7%, which was likely to underestimate the incidence of AIV infection in the sampled birds because of pooling of swabs. Over the same time period only a single virus was isolated from birds sampled at other locations in Ireland. The overall AIV detection rate in waterfowl in Ireland was relatively low compared with similar studies elsewhere [Reference Munster9, Reference Hanson34, Reference Olsen35]. However, those studies were conducted at locations nearer to the breeding grounds of migratory waterfowl [Reference Olsen35, Reference Parmley36] and previous studies in North America have demonstrated an inverse relationship between the AIV detection rate in waterfowl and the distance of the sampling site from their breeding grounds [Reference Hanson34]. Nine distinct subtypes of AIV, H6N2, H10N7, H2N3, H11N9, H6N5, H6N6, H10N2, H10N5 and H10N6 were isolated from waterfowl at the Sloblands over four winters. The detection of most AIV isolates in November, the range of subtypes and the predominance of a different subtype amongst the viruses isolated during two consecutive seasons suggested annual introduction of new viruses to the Sloblands. Migratory waterfowl are the most likely means of introduction of new viruses, and as birds from both Northern Europe and North America [Reference Wernham27, Reference Crowe and Merne28] over-winter at the Sloblands, it is possible that AIV might have been introduced from either region. The long distance travelled by migrants from the northwest, mainly light-bellied Brent geese (Branta bernicla hrota) from the east Canadian Arctic and white-fronted geese (Anser albifrons flavirostris) from Greenland [Reference Wernham27, Reference Crowe and Merne28] may result in clearance of AIV from these birds before their arrival in Ireland. AIV was not isolated from any of the 100 Brent and 25 white-fronted geese sampled, including four Brent and 23 white-fronted geese at the Wexford Sloblands (Table 2). Barnacle geese (Branta leucopsis) from Greenland also winter in Ireland but mostly on the west coast and very few are found on the Wexford Sloblands [Reference Wernham27, Reference Crowe and Merne28]. The farmed mallard were not tested for AIV prior to release, so it is also possible that these birds may have been responsible for the introduction of some AIVs to the ecosystem at the Sloblands. However, as the mallard had been reared outdoors, the origin of any AIVs detected would have been difficult to ascertain. Furthermore, given the similarity between the range of viral subtypes found in the waterfowl sampled at the Sloblands and those sampled at other locations in Northern Europe over the same time period [Reference Munster9], it is most likely that new AIVs are introduced to Ireland by duck species migrating along the east Atlantic Flyway. Seven of the isolates were obtained from wigeon and pintail ducks, winter visitors from Northern Europe [Reference Wernham27, Reference Crowe and Merne28]. The evidence suggested that sedentary mallard played a major role in the amplification of the AIVs introduced by migrating species.
Ecological factors largely explain the higher AIV detection rates in waterfowl at the Sloblands compared with other sites in Ireland. The influx of migratory waterfowl, coupled with the annual release of hand-reared mallard prior to the shooting season ensured a very high density of susceptible birds during the winter months. Supplementary feeding of the mallard further encouraged congregation of waterfowl, thus increasing the degree of contact and the opportunity for transmission of AIV. The unique landscape of the Sloblands, consisting of low-lying polders traversed by a wide shallow channel, is likely to have facilitated viral spread. Heavy faecal contamination of this semi-stagnant body of water, the long duration of infectivity of AIVs in lake water [Reference Parmley36, Reference Stallknecht37] and the filter-feeding behaviour of dabbling ducks provided optimal conditions for virus transmission [Reference Munster9].
Methodological factors may also have influenced the likelihood of detecting AIV. The relatively high virus detection rate at the Sloblands may, to some extent, reflect the age profile of sampled birds because the rate of AIV recovery in waterfowl is inversely proportional to the age of the birds sampled [Reference Hanson34–Reference Parmley36, Reference Stallknecht and Shane38]. Juvenile birds tend to be over-represented in a sample of waterfowl that has been shot in flight and the vast majority of the mallard at the Sloblands were birds less than 1-year-old that had been reared in captivity and released prior to the hunting season. Juvenile birds were also predominant amongst the teal and wigeon sampled at the Sloblands. Therefore, the detection rate in the Sloblands was likely to have been greater because of this bias towards juveniles in the sample.
The handling and storage of specimens are also likely to influence detection rates of AIV, especially when VI is used. In the present study, this may have contributed in part to the difference in AIV detection rates between the Sloblands and other sites in Ireland. All of the birds at the Sloblands were sampled by experienced personnel within a short time of death. Furthermore, these specimens were chilled quickly, pending delivery to the laboratory. On the other hand, in the case of swabs collected by hunters and the carcases of dead birds submitted by members of the public, viral survival and, to a lesser degree, RNA integrity may have been compromised by poor handling and storage. Based on the evidence of this study, virus isolates were unlikely to be obtained unless swabs were placed in VTM soon after sampling, chilled quickly and returned immediately to the laboratory for storage under optimal conditions. All isolates in this project were obtained from samples where the VTM had been maintained at +4°C during transport. Nonetheless, 327/650 waterfowl sampled by the project team, using best practice, at locations outside of the Wexford Sloblands were negative for AIV. Furthermore, the only AIV isolated outside of the Sloblands was obtained from a shelduck in a coastal wildlife park where there was a large collection of diverse species of waterfowl. The highest percentage of rtRT–PCR-positive specimens from hunted birds that were sampled after January 2006 was also obtained in the Sloblands. No AIV was detected in specimens from wild birds sampled at non-coastal locations. Furthermore, AIV was not detected in species other than aquatic birds (data not included). These findings demonstrated that scattered unfocused surveillance of birds in Ireland for AIV provided very little material with which to study the gene pool of influenza viruses, while contributing significantly to laboratory and field costs in terms of finance and personnel. Rationalization of sampling programmes, in terms of species and location, would ensure better use of resources and provide a greater source of material for genetic and epidemiological studies.
None of the viruses isolated in the present study were of the H5 or H7 subtypes and consequently all were assumed to be of low pathogenicity. However, two specimens collected from teal were confirmed as LPAI H5 virus by molecular methods. Therefore, there is a risk that low-pathogenic H5 or H7 viruses, which can subsequently mutate from LPAI to HPAI biotypes [Reference Kawaoka13], may be introduced into poultry flocks through contact with waterfowl [Reference Campitelli39]. The failure to detect highly pathogenic AIV in waterfowl in Ireland and extensive serosurveillance on Irish poultry farms over the same period has demonstrated that there is no evidence of HPAI virus circulation in domestic poultry, although viruses of low pathogenicity were isolated from farmed birds at two different locations in Ireland during the course of this study [11]. Whereas neither of these premises was located near to the Sloblands, a strong epidemiological link to waterfowl populations was identified in each case. In general, the findings of this study provide considerable reassurance to the poultry industry in Ireland that the risk of avian influenza is low. Because of the low incidence of AIV detected in waterfowl, there is a reduced risk of transmission of influenza viruses to free-range poultry and subsequent spread within the industry. However, the results also demonstrate the value of continuing surveillance of waterfowl populations in order to detect periods of high incidence of H5 or H7 AIVs in free-living birds. Additional precautionary measures could be implemented during periods of increased circulation of AIV. The timely provision to the poultry industry of data on AIV circulation combined with increased surveillance testing of domestic birds to detect LPAI viruses, before there is an opportunity for mutation to virulence, could help to prevent outbreaks of HPAI.
This is the first published study on the incidence and characterization of AIVs in wild birds in Ireland. It has established that waterfowl in Ireland harbour diverse subtypes of AIV and that the most likely origin of these is Northern Europe. However, circulation of these viruses in Ireland is likely to be concentrated in suitable ecological pockets whereas the general incidence throughout the country is relatively low. In global terms, countries with similar geographical features to Ireland have less risk of avian influenza outbreaks than countries in Northern and Eastern Europe, where a higher incidence of AIV was found in wild birds [Reference Munster9, Reference Olsen35]. Molecular analysis of the isolates obtained may provide definitive confirmation of their origin. These isolates can now be used in transmission studies to increase our knowledge of the pathogenicity and epidemiology of LPAI viruses. The study revealed little evidence of the circulation of H5 and H7 subtypes of AIV in Ireland. No H5N1 HPAI viruses were detected despite their presence in European countries during this period [Reference Pittman26] and thus, there was no evidence of long-distance transmission of these viruses. Nevertheless, the identification of numerous other AIV subtypes in waterfowl and the contemporaneous occurrence of AIV infection in farmed birds linked to waterfowl populations emphasize the need for continued vigilance. Sites such as the Sloblands, with a high density of waterfowl, provide ideal conditions for the circulation of AIVs. Therefore, care in the location of new poultry enterprises to avoid these ‘high risk’ areas should reduce the chances of outbreaks of avian influenza. Continued targeted sampling at such sites would be a cost-effective method to provide early warning of the circulation of new subtypes of AIV in waterfowl, while dabbling ducks, including juvenile mallard released into the wild by hunters, could usefully be targeted as sentinels.
ACKNOWLEDGEMENTS
The support and encouragement of Mr Pat Lenihan, Dr John Ferris and Dr P. J. O'Reilly at the Central Veterinary Research Laboratory is gratefully acknowledged. We also gratefully acknowledge the assistance provided during the collection of specimens by members of the National Association of Regional Game Councils and of the National Parks and Wildlife Service.
DECLARATION OF INTEREST
None.









