Introduction
Major depressive disorder (MDD) is typically a chronic disease characterized by recurrent episodes and lingering residual symptoms.Reference Malhi and Mann 1 , Reference Cosci, Mansueto and Fava 2 Patients with recurrent episodes of depression are likely to have increased rates of morbidity and disability, poorer long-term prognosis, and more suicide attempts. 3 Moreover, a majority of depressed patients relapse within a year and become treatment-resistant.Reference Fava and Davidson 4 Patients suffering from treatment-resistant depression (TRD) experience significant cognitive impairment, increased morbidity and suicidal risk, which can impose higher socioeconomic burden.Reference Fava and Davidson 4 , Reference Russell, Hawkins and Ozminkowski 5 According to the Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-5), MDD is diagnosed if at least five out of its nine symptoms (depressed mood; anhedonia; feelings of worthlessness/guilt; suicidality; fatigue/anergia; insomnia or hypersomnia; decreased or increased appetite/weight; decreased ability to think or concentrate or make decisions; and psychomotor agitation or retardation) co-occur for at least 2 weeks and cause clinically significant distress and/or functional impairment. 3 It is also important to exclude other conditions with similar symptomatology to confirm the diagnosis. 3
Current Pharmacotherapies for Depression
Several interventions including pharmacotherapy, psychotherapy, neuromodulation including electroconvulsive therapy (ECT) and transcranial magnetic stimulation (TMS), light therapy, neurosurgical, and lifestyle interventions (regular physical exercise, healthy diet, and sleep hygiene) exist for the treatment of MDD.Reference Cuijpers, Karyotaki and Eckshtain 6 , Reference Cook, Espinoza and Leuchter 7 Out of these treatment modalities, pharmacotherapy with antidepressant drugs is the most frequently used intervention.Reference Dupuy, Ostacher and Huffman 8
Several classes of antidepressant agents are currently available for treatment of MDD.Reference Nierenberg 9 , Reference Baldessarini, Brunton, Lazo and Parker 10 Classical antidepressant types include monoamine oxidase inhibitors (MAOIs) such as tranylcypromine and selegiline, and tricyclic antidepressants (TCAs) such as imipramine and amitriptyline. This was followed by the introduction of other classes of drugs including Selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine, citalopram, sertraline, serotonin/norepinephrine reuptake inhibitors (SNRIs) such as venlafaxine, duloxetine, milnacipran; norepinephrine/dopamine reuptake inhibitors (NDRIs), notably bupropion; serotonin antagonist-reuptake inhibitors (SARIs) such as trazadone, agents with indirect noradrenergic and serotonergic actions (NaSSAs), notably mirtazapine; and agents with multimodal serotonergic targets (vilazodone and vortioxetine). The efficacy of most of these agents is mainly attributed to their enhancing effects on monoaminergic functions by various mechanisms such as inhibition of the serotonin (5-HT) and/or norepinephrine (NE) and/or dopamine (DA) reuptake, increasing the firing rates of 5-HT and NE neurons and modulation of the 5-HT system via direct effects on different 5-HT receptors.Reference Taylor, Fricker, Devi and Gomes 11 , Reference Harmer, Duman and Cowen 12
Adverse effects commonly associated with antidepressant treatment can contribute to poor treatment adherence. MAOIs and TCAs are lethal in overdose due to cardiac toxicity.Reference Baldessarini, Brunton, Lazo and Parker 10 Nausea, sexual dysfunction, insomnia, somnolence, fatigue, and weight-gain are common adverse events associated with SSRIs and SNRIs.Reference Hu, Bull and Hunkeler 13 , Reference Thase, Haight and Richard 14 In addition, abrupt cessation of SSRIs and SNRIs can result in the emergence of antidepressant withdrawal syndrome with symptoms of dizziness, nausea, anxiety, and irritability.Reference Sir, D’Souza and Uguz 15 All of these and other adverse events of available antidepressants prompt the search for new molecular targets and unique brain pathways that extend beyond the typical monoamine targets and pathways, which can guide the development of novel antidepressant drugs with faster onset of action, and improved efficacy, tolerability, and long-term effectiveness than currently available antidepressants.
Glutamatergic Hypothesis of Depression
The glutamatergic system is increasingly becoming the primary mediator in the background of psychiatric pathology, and specifically a newly emerging major target underlying the pathophysiology of depression and beyond. Glutamate has been implicated in association with MDD following observations of altered glutamate and glutamine levels in brain, cerebrospinal fluid (CSF), plasma and serum in mood disorder patients.Reference Sanacora, Treccani and Popoli 16 Higher glutamate plasma levels in depressed patients and higher glutamate levels in the frontal cortex of deceased depressed patients have been reported.Reference Hashimoto, Sawa and Iyo 17 , Reference Sanacora, Zarate, Krystal and Manji 18 Postmortem studies found altered mRNA and protein expression in ionotropic and metabotropic glutamate receptors in depression-relevant brain areas in MDD patients, pointing to a role of dysregulated glutamate signaling in the pathophysiology of MDD.Reference Beneyto, Kristiansen and Oni-Orisan 19 Animal studies have also supported the hypothesis that stress, which has a well documented role in the development of depression, activates glucocorticoid signaling enhancing glutamate release in brain areas including the amygdala, hippocampus, and prefrontal cortex, and leading to functional and morphological changes in rodent models including atrophy, retraction, and simplification of dendritic arbor also contributing to observed hippocampal and PFC volumetric changes in MDD also observed in humans.Reference Sanacora, Treccani and Popoli 16 , Reference Koolschijn, van Haren and Lensvelt-Mulders 20 The increasing amount of studies and observations led to the articulation of the glutamate hypothesis of depression, which postulates that alterations in the release, metabolism, and clearance of glutamate leads to its sustained accumulation and consequential structural and functional changes coupled with impaired synaptic activity in depression-relevant brain areas associated with mood regulation and related cognitive and emotional changes (Table 1).Reference Musazzi, Treccani and Popoli 21
Table 1. Novel Antidepressant Drugs: Dose, Efficacy, and Adverse Events
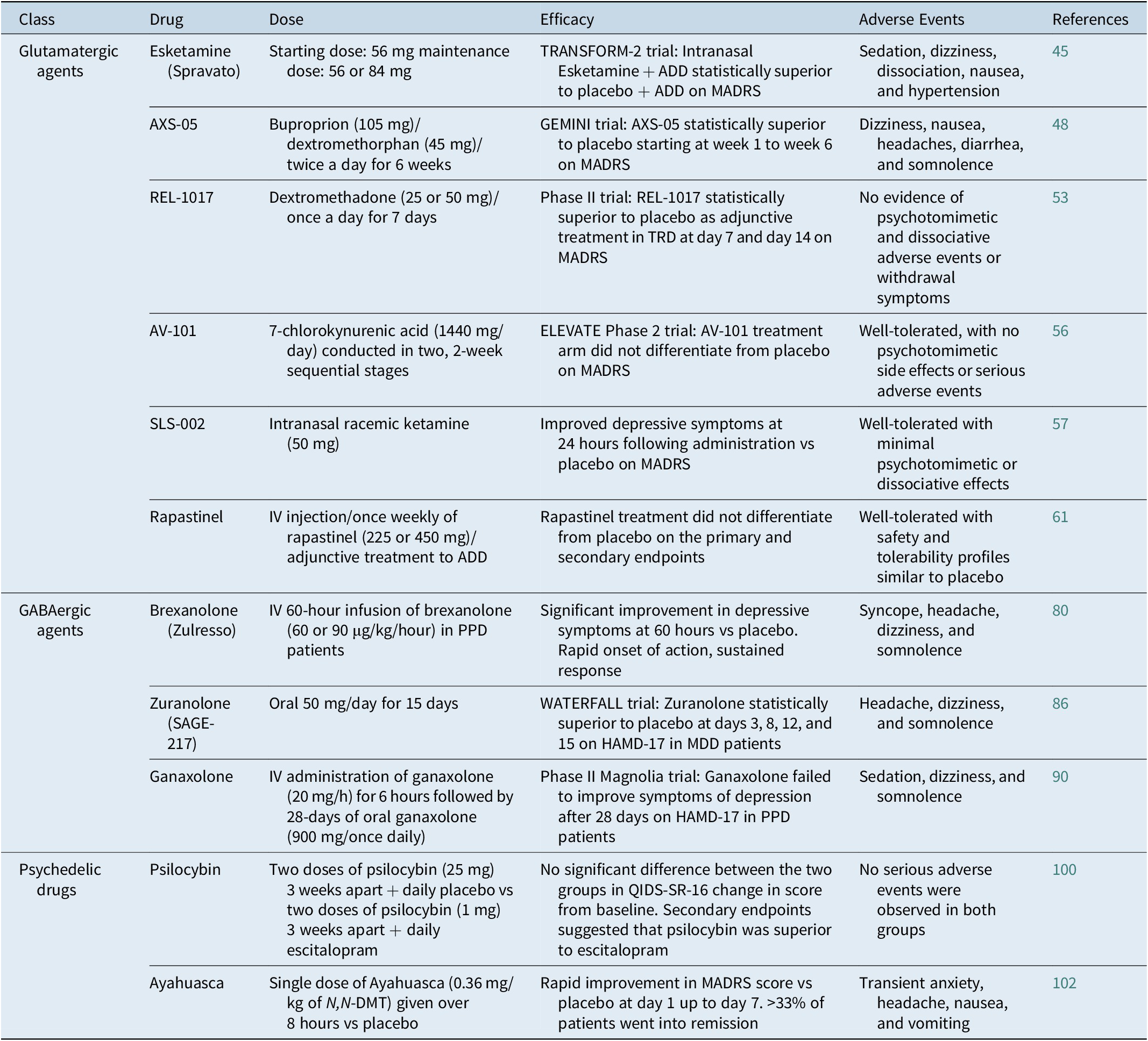
Abbreviations: ADD, antidepressant drug; HAMD-17, 17-item Hamilton rating scale for depression; IV, intravenous; MADRS: Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; PPD, postpartum depression; QIDS-SR-16, 16 item quick inventory of depressive symptomatology-self R.
Ketamine
Ketamine was developed in 1962 as a safer and short-acting alternative anesthetic molecule to phencyclidine producing dissociative anesthesia and effective analgesia without respiratory depression,Reference Hashimoto 22 and later also became a popular recreational drug due to its psychomimetic effects. Ketamine is a prototypic glutamatergic modulator which also has sigma-1 and μ opioid activating properties, and has recently become the model molecule for a novel approach to antidepressant action due to its reproducible rapid and robust antidepressive, antianhedonic, and antisuicidal effects after a single subanaesthetic dose.Reference De Berardis, Tomasetti and Pompili 23 , Reference Hausman, Meffert, Mosich and Heinz 24
Several action mechanisms have been proposed for ketamine including glutamatergic, opioidergic, and neurotrophic targets,Reference Krystal, Sanacora and Duman 25 which supports the neuroplasticity and glutamatergic hypothesis of MDD and the role of disrupted structure and function of the glutamate circuitry associated with stress-related psychopathology.Reference Duman, Aghajanian, Sanacora and Krystal 26 Ketamine’s antidepressant effects are potentially mediated by direct and indirect inhibition of NMDA receptors, disinhibition of GABA interneurons, as well as conversion to hydroxy-norketamine (HNK) during metabolism, which enhances presynaptic glutamate release and glutamatergic throughput at AMPA receptors leading to sustained excitatory synapse potentiation in the background of maintained antidepressant response.Reference Zanos and Gould 27 Several additional studies have suggested that Brain-Derived Neurotrophic Factor (BDNF) and its receptor, tropomyocin receptor kinase B (TrkB), the mTORC1 (mammalian target of rapamycine complex 1), and the mitogen activated protein kinase (MAPK) signaling pathway are crucial mediators of the antidepressant effects of ketamine.Reference Yang, Yang, Luo and Hashimoto 28 , Reference Abdallah, Averill and Gueorguieva 29 A small clinical trial suggested that the rapid antidepressant effects of ketamine requires the activation of the opioid system since pretreatment of TRD patients with naltrexone (nonselective opioid receptor antagonist) blocked the antidepressant effects of ketamine.Reference Williams, Heifets and Blasey 30
Ketamine exerted its antidepressant effects in single subanesthetic IV doses,Reference Berman, Cappiello and Anand 31 leading to rapid robust and sustained effects in TRD patients, with a 70% response rate at 24 hours.Reference Zarate, Singh and Carlson 32 , Reference Zarate, Brutsche and Ibrahim 33 Ketamine also rapidly reduced suicidal thoughts within a few hours following administration and lasting up to 7 days in severely depressed patients with suicidal ideation.Reference Wilkinson, Ballard and Bloch 34 Ketamine’s rapid and robust efficacy is mainly shadowed by its psychotomimetic and dissociative side effects as well as its abuse liability, which encouraged a search for agents that are comparable NMDA receptor antagonists but without the above adverse effects.Reference Kadriu, Musazzi and Henter 35 However, understanding the processes in the background of ketamine’s mechanism of action paves the way for the development of novel glutamatergic therapeutic agents and targets as well as repurposing of older agents, especially with the aim of finding molecules that retain the antidepressant properties of ketamine without its use-limiting adverse effects.Reference Kadriu, Musazzi and Henter 35
Esketamine
In 2019, the US Food and Drug Administration (FDA)-approved esketamine (Spravato) nasal spray in conjunction with an oral antidepressant for the treatment of adult TRD patients. Esketamine, a noncompetitive NMDA receptor antagonist, offered the first novel mechanism of action for an antidepressant drug in over 30 years. It is also the first antidepressant drug classified as a Schedule III controlled substance (CIII), and can be assoicated with abuse and diversion.Reference Kryst, Kawalec and Pilc 36 Esketamine has been found to be effective in several randomized, controlled, multicentre Phase 2 trials in TRD patients administered in a single infusion,Reference Correia-Melo, Leal and Carvalho 37 , Reference Singh, Fedgchin and Daly 38 or after intranasal administration as an add-on treatment to existing antidepressants.Reference Daly, Singh and Fedgchin 39 , Reference Daly, Trivedi and Janik 40 Other clinical trials (ASPIRE I and II) demonstrated rapid improvement in measures of suicidal ideation among depressed patients at imminent risk for suicide.Reference Canuso, Singh and Fedgchin 41 Three randomized, placebo-controlled Phase 3 studies of 4 weeks of intranasal ketamine as an add-on treatment (TRANSFORM-1, TRANSFORM-2, and TRANSFORM-3), in addition to long-term randomized withdrawal studies for relapse prevention (SUSTAIN-1) and long-term safety (SUSTAIN-2) also confirmed efficacy and manageable safety profile as well as reduced relapse risk and sustained improvement for up to 52 weeks in TRD patients.Reference Daly, Trivedi and Janik 40 , Reference Fedgchin, Trivedi and Daly 42 -Reference Wajs, Aluisio and Holder 44
Esketamine showed a more favorable side effect profile compared to ketamine but worse compared to placebo,Reference Singh, Fedgchin and Daly 38 , Reference Daly, Singh and Fedgchin 39 with most frequent adverse effects including dissociation, nausea, vertigo, dysgeusia, and dizziness in more than 20% of patients when used as an add-on treatment in TRD. Less frequent side effects include anxiety, lethargy, hypoesthesia, vomiting, feeling drunk, elevation in blood pressure and heart rate, and confusion with mild to moderate severity, which are mostly transient appearing on the day of administration and resolving on the same day lasting 4 to 6 hours and correlated with serum levels.Reference Daly, Singh and Fedgchin 39 , Reference Canuso, Singh and Fedgchin 41 -Reference Ochs-Ross, Daly and Zhang 43 Due to the risk for sedation and dissociation, the potential for abuse or misuse, and the possibility of increases in blood pressure, esketamine nasal spray is only available through a restricted Risk Evaluation and Mitigation Strategy (REMS) distribution system. Patients self-administer Esketamine nasal spray only in REMS-certified healthcare settings, and need to be monitored for at least 2 hours after drug administration.Reference Bahr, Lopez and Rey 45
AXS-05
AXS-05 is a proprietary fixed dose combination of bupropion and dextrometorphan (DM) with multimodal activity. DM is a low affinity NMDA antagonist, sigma-1 agonist, SNRI with limited bioavailability,Reference Nguyen, Thomas and Lucke-Wold 46 while bupropion is a NE and DA reuptake inhibitor, which helps to increase the bioavailability of DM. In Phase II ASCEND clinical trial, AXS-05 showed significant improvement of depressive symptoms at 6 weeks of treatment compared to bupropion in MDD patients, with 47% of patients achieving remission vs. 16% on buproprion alone. The effects were rapid, with AXS-05 showing superiority to bupropion as early as week 1 throughout week 6. Most common adverse effects reported in the AXS-05-treated patients were nausea, dizziness, dry mouth, decreased appetite, and anxiety. No psychotomimetic effects or metabolic deficits were associated with AXS-05 treatment. 47 In Phase III GEMINI clinical trial, AXS-05 demonstrated rapid and sustained improvement in depressive symptoms compared to placebo. AXS-05 treatment prolonged remission and improved disease severity, functional impairment and quality of life of MDD patients. 48 A more recent study (COMET-SI trial) reported that AXS-05 reduced suicidality score, resolved suicidal ideation in 60% of MDD patients who have suicidal ideation by week 1 and 78% by week 4, and improved functioning in 51% of MDD by week 1 and 77% by week 6. 49
REL-1017
Dextromethadone (REL-1017) is the d-stereoisomer of methadone acting as a noncompetitive NMDA receptor antagonist with a preference for pathologically hyperactive channels,Reference Fogaca, Fukumoto and Franklin 50 granted fast track designation by the FDA as an adjunctive treatment for depression. Its parent drug, methadone, is a synthetic racemic mixture used for the treatment of opioid dependence as well as severe pains exploiting its agonistic effects on μ receptors.Reference Bernstein, Davis and Mills 51 Both stereoisomers of methadone bind MK-801 sites on the NMDA receptor complex. However, the d-stereoisomer shows a 10 to 30-fold lower affinity for opioid μ and δ receptors,Reference Gorman, Elliott and Inturrisi 52 and therefore a lack of opioid-like effects in doses that exhibit antidepressant activity.Reference Bernstein, Davis and Mills 51 d-methadone shows antidepressant effects with convergent signaling and synaptic mechanisms with ketamine. Administration of a single dose of methadone stimulated mTORC1 signaling, enhanced synaptic function in the medial prefrontal cortex (mPFC) and increased BDNF release. These findings suggest that d-methadone induces rapid antidepressant effects in different behavioral models through mTORC1-mediated synaptic plasticity in the mPFC similar to ketamine.Reference Fogaca, Fukumoto and Franklin 50
In two Phase I studies linear pharmacokinetics was observed up to 150 mg single doses and up to 75 mg daily doses for up to 10 days without severe adverse events, no psychotomimetic, or dissociation symptoms, no withdrawal or discontinuation symptoms, and no respiratory depression.Reference Bernstein, Davis and Mills 51 Phase II trial reported that both doses (25 and 50 mg) of REL-1017 significantly improved depressive symptoms as adjunctive treatment in TRD patients as early as day 4 through Day 7 and at Day 14, 7 days after treatment discontinuation. 53 The study also confirmed the favorable safety and tolerability profile of REL-1017, as there was no evidence of treatment-induced psychotomimetic and dissociative adverse events or withdrawal symptoms upon treatment discontinuation. There are two ongoing double-blind, placebo-controlled phase 3 clinical trials (RELIANCE I and RELIANCE II) assessing the efficacy and safety of REL-1017 once daily as an adjunctive treatment of MDD, and one long-term, open-label safety study for REL-1017 (RELIANCE-OLS), which will include both patients continuing from the pivotal studies as well as new enrolled MDD patients. 54
AV-101
AV-101 is a prodrug that readily crosses the blood-brain barrier and is converted to 7-chlorokynurenic acid (7-CI-KYNA), which blocks the glycineB co-agonist site of the NMDA receptor.Reference Zanos, Piantadosi and Wu 55 It displayed rapid and persistent antidepressant effects in animal models for depression and without the rewarding and psychotomimetic effects of ketamine.Reference Zanos, Piantadosi and Wu 55 A Phase 2 double-blind, placebo-controlled trial (ELEVATE study) evaluated the efficacy, safety, and tolerability of AV-101 as an adjunctive treatment in 199 MDD patients who failed to respond adequately to standard antidepressant therapy. In this study, AV-101 treatment failed to differentiate from placebo on the Montgomery-Åsberg Depression Rating Scale (MADRS) and did not improve symptoms of MDD. 56
SLS-002
SLS-002 is an intranasal racemic ketamine formulation in clinical development as a novel therapy for suicidal ideation and behavior in MDD patients. An earlier clinical trial reported that intranasal ketamine (50 mg) was effective in improving depressive symptoms at 24 hours following administration compared to placebo on MADRS. Intranasal ketamine was well-tolerated with minimal psychotomimetic or dissociative effects.Reference Lapidus, Levitch and Perez 57 Seelos Therapeutics is evaluating the efficacy, safety, and tolerability of SLS-002 in addition to standard of care on symptoms of MDD and suicidality in 136 patients who are assessed to be at imminent risk for suicide, as measured by the change from baseline on the MADRS total score at 24 hours post first dose (ClinicalTrials.gov Identifier: NCT04669665).
Rapastinel and AGN241751
Rapastinel (GLYX-13) is a centrally active, IV-administered amidated tetrapeptide (Thr-Pro-Pro-Thr-NH2) that acts as a selective, weak partial agonist of an allosteric site of the glycine site of the NMDA receptor complex. Preclinical studies reported that rapastinel displayed rapid-acting and long-lasting antidepressant effects, robust cognitive-enhancing effects without psychotomimetic side effects in several animal models for depression and cognition.Reference Burgdorf, Zhang and Nicholson 58 , Reference Burgdorf, Zhang and Weiss 59 A Phase 2 double-blind, randomized, placebo-controlled study reported that IV administration of rapastinel (5 or 10 mg/kg) reduced depressive symptoms within 2 hours and this effect was maintained for 7 days on average in MDD patients who had not responded to another antidepressant drug.Reference Preskorn, Macaluso and Mehra 60 Three Phase III double-blind, randomized, placebo-controlled trials assessed the efficacy, safety and tolerability of rapastinel as adjunctive treatment in MDD patients who had a partial response to previous antidepressant drugs. In all three pivotal clinical trials, rapastinel was well-tolerated and demonstrated a safety and tolerability profile similar to placebo. However, rapastinel failed to improve symptoms of depression and to differentiate from placebo in all phase III trials. Interim analysis of fourth relapse prevention study suggested the primary endpoint will not be met. As a result, the development of rapastinel was discontinued. 61
AGN-241751 is a novel orally bioavailable, small molecule NMDA receptor modulator with similar mechanism of action to rapastinel. Preclinical studies showed that AGN-241751 exerts antidepressant-like effects and reverses behavioral deficits induced by chronic unpredictable stress in mice by activating mTORC1 signaling and increasing levels of synaptic proteins crucial for synaptic plasticity in mPFC.Reference Pothula, Liu and Wu 62 Ongoing double-blind, placebo-controlled trials are evaluating the efficacy and safety of multiple doses of AGN-241751 in adult MDD patients.
PCN-101 (arketamine)
PCN-101 is the R-isomer of ketamine that is being developed for therapeutic treatment of psychiatric illnesses such as TRD. Preclinical studies suggested that R-ketamine has more durable and potent effects than Esketamine despite a lower affinity to the NMDA receptor and potentially a more favorable safety and tolerability profile.Reference Yang, Shirayama and Zhang 63 A clinical study that evaluated the safety, tolerability, and pharmacokinetics of single ascending IV doses of PCN-101 in 58 healthy adult volunteers found that PCN-101 was safe and well-tolerated at all doses up to 150 mg, the highest dose tested. 64 An open-label pilot trial reported that IV infusion of R-ketamine to seven TRD patients produced fast-onset and sustained antidepressant effects with favorable safety and tolerability profiles.Reference Leal, Bandeira and Correia-Melo 65 Ongoing trials are further evaluating the efficacy, safety, and tolerability of PCN-101 in TRD patients.
GABAergic Hypothesis of Depression
γ-aminobutyric acid (GABA) is the principal inhibitory neurotransmitter in the CNS. GABAergic neurons exit as local interneurons or they can project to connect distant brain areas with each other. The GABAergic system influences various other neurotransmitter systems implicated in the pathophysiology of depression, and controls the activities of the 5-HT and NE neurons of the raphe nuclei and locus coeruleus. In addition, GABAergic signaling also controls the hypothalamic-pituitary-adrenal axis (HPA) at the level of the paraventricular nucleus of the hypothalamus.Reference Watanabe, Maemura and Kanbara 66 GABA mediates its actions via ionotropic (GABAA and GABAC) and metabotropic (GABAB) receptors. GABAA receptors are five subunits comprising ligand-gated ion (chloride) channels. The subunit composition of the GABAA receptor determines its pharmacological and physiological signatures.Reference Sigel and Steinmann 67
The implication of the GABAergic system in the pathophysiology of MDD stems from several in vivo spectroscopy or postmortem studies, which reported lower GABA levels in several cortical areas, diminished levels of GABA-synthesizing enzymes, and decreased GABA levels in the cerebrospinal fluid and plasma of depressed patients or suicide victims.Reference Sanacora, Mason and Rothman 68 -Reference Luscher, Shen and Sahir 70 In addition, treatment with antidepressant drugs, ECT or TMS was associated with an increase in GABA concentrations in depressed patients, suggesting that normalization of GABA deficits in depressed patients may contribute to the beneficial therapeutic effects of pharmacotherapies and neuromodulatory interventions used for treatment of MDD and TRD.Reference Sanacora, Mason, Rothman and Krystal 71 -Reference Dubin, Mao and Banerjee 73 The GABAergic system has recently attracted attention as a brain pathway that constitutes a novel target for developing new antidepressants with unique mechanisms of action that extend beyond the traditional monoamine targets.
Brexanolone
Brexanolone (Zulresso) is an intravenous (IV) formulation of the neurosteroid allopregnanolone, which acts as a “positive allosteric modulator” (PAM) of the GABAA receptors and is able to bind both synaptic and extrasynaptic GABAA receptors.Reference Akk, Covey and Evers 74 Decreased levels of allopregnanolone in the plasma or CSF are associated with increased risks of depression and anxiety in adult patients.Reference Schule, Nothdurfter and Rupprecht 75 In addition, lower levels of allopregnanolone during pregnancy can increase the risk of postpartum depression (PPD).Reference Osborne, Gispen and Sanyal 76 In MDD patients, treatment with antidepressant drugs normalized CSF allopregnanolone levels, which significantly correlated with the improvement in depressive symptoms.Reference Uzunova, Sheline and Davis 77 In addition, allopregnanolone display anti-inflammatory and neurogenesis promoting properties, making it an attractive antidepressant/anxiolytic agent.Reference Evans, Sun, McGregor and Connor 78
Brexanolone was evaluated as a novel treatment for PPD in several clinical trials. An open-label study found that a single 60-hour IV infusion of brexanolone had rapid antidepressant effects in severe PPD patients with good safety and tolerability profiles.Reference Kanes, Colquhoun and Doherty 79 Two additional double-blind, placebo-controlled, phase 3 trials randomized severe PPD patients to receive a single IV 60-hour infusion of either brexanolone (60 or 90 μg/kg/hour) or matching placebo. Administration of brexanolone resulted in profound improvement in depressive symptoms at 60 hours compared with placebo, with rapid onset of action and sustained treatment response. The most common adverse events were headache, dizziness, and somnolence. Two patients reported serious adverse effects including suicidal ideation, altered state of consciousness and syncope.Reference Meltzer-Brody, Colquhoun and Riesenberg 80 Based on the efficacy and safety results of the above studies, brexanolone was approved by the FDA at March 19, 2019 as the first treatment of PPD in adult women. 81 FDA, however, had concerns about serious risks, including excessive sedation or sudden loss of consciousness during administration, and approved brexanolone with a Risk Evaluation and Mitigation Strategy (REMS) and it is only available to patients through a restricted distribution program at certified health care facilities where patients can be carefully monitored throughout the duration of treatment. 81
Zuranolone (SAGE-217)
Zuranolone (SAGE-217) a synthetic neuroactive steroid with potent PAM activity on both synaptic and extrasynaptic GABAA receptors. Zuranolone exhibited pharmacokinetic properties with long half-life, which facilitate once daily oral dosing, and produced robust pharmacodynamic effects consistent with in vivo GABAergic activity following administration.Reference Althaus, Ackley and Belfort 82 Zuranolone also increased tonic and phasic GABA currents, which reflects increased trafficking of extrasynaptic GABAA receptors.Reference Abramian, Comenencia-Ortiz and Modgil 83
A Phase 2 clinical trial randomly assigned 89 MDD patients to receive zuranolone (30 mg) or placebo once daily. Administration of zuranolone daily for 14 days resulted in a profound reduction in depressive symptoms at day 15. The most common adverse side effects in patients receiving zuranolone were headache, dizziness, nausea, and somnolence.Reference Gunduz-Bruce, Silber and Kaul 84 A pivotal Phase 3 clinical trial (MOUNTAIN study) randomized 581 MDD patients to receive SAGE-217, 20 or 30 mg, or placebo, once-nightly for 2-weeks. Both doses of zuranolone failed to achieve statistical significance compared to placebo at day 15 (primary endpoint). However, the higher dose (30 mg) of zuranolone significantly improved depressive symptoms at days 3, 8, and 12. Zuranolone was well-tolerated with safety profile comparable to placebo. Headache, dizziness, and somnolence were the main side effects. 85 A more recent pivotal Phase 3 study (WATERFALL study) reported that zuranolone at a higher dose (50 mg/day) showed statistically significant improvement in depressive symptoms in MDD patients vs placebo at day 15 as assessed by the 17-item Hamilton Rating Scale for Depression (HAMD-17) total score. Rapid onset of treatment effect was observed on days 3, 8, and 12 on HAMD-17. Zuranolone was well-tolerated and demonstrated a safety profile consistent with previous clinical studies. 86
The efficacy and safety of zuranolone (30 mg/day) were also evaluated in a 2-week, double-blind, placebo-controlled Phase 3 trial (ROBIN study), which enrolled 151 adult female patients diagnosed with severe PPD. Zuranolone significantly improved the depressive symptoms in PPD patients, with good safety and tolerability profiles. The significant antidepressant effects of zuranolone were detected at day 3 through 2-weeks of treatment, and maintained for 4 weeks after treatment.Reference Frieder, Fersh, Hainline and Deligiannidis 87 Additional clinical trials are ongoing to further evaluate the efficacy, safety and tolerability of zuranolone in MDD and PDD patients.
Ganaxolone
Ganaxolone is a synthetic 3β-methylated derivative of allopregnanolone that acts as a PAM of GABAA receptors and binds to synaptic and extrasynaptic GABAA receptors.Reference Carter, Wood and Wieland 88 A small open-label uncontrolled pilot study trial evaluated the effects of ganaxolone as adjunctive therapy for persistent depression despite adequate antidepressant treatment in 10 postmenopausal women. At the end of the 8-week study period, 44% of participants showed improvement and went into remission. Dizziness, sleepiness, and fatigue were common side effects in all participants.Reference Dichtel, Nyer and Dording 89 A Phase 2 (Magnolia) clinical trial reported that IV administration of ganaxolone (20 mg/h) for 6 hours followed by 28-days of oral ganaxolone (900 mg once daily) in women with PPD failed to improve symptoms of depression after 28 days of treatment on HAMD-17. Ganaxolone was safe and well-tolerated. The most common reported adverse events were sedation, dizziness, and somnolence. There were no reports of syncope or loss of consciousness. 90 Ganaxolone is being investigated for potential medical use in the treatment of different types of epilepsy and rare genetic disorders including CDKL5 deficiency disorder and tuberous sclerosis complex.
Psychedelic Drugs as Novel Treatments for MDD
Medical psychedelic research has undergone a renaissance in recent years. The momentum to evaluate the clinical benefits of psychedelic drugs in different debilitating neuropsychiatric disorders and other conditions, including MDD and TRD, anxiety and depressive symptoms in advanced cancer patients, alcohol and nicotine dependence, obsessive compulsive disorder (OCD), anorexia nervosa, and post-traumatic stress disorder (PTSD), has increased exponentially.Reference Nutt and Carhart-Harris 91 , Reference Nutt, Erritzoe and Carhart-Harris 92 Psychedelics include plant medicines such as psilocybin [(4-phosphoroyloxy-N,N-dimethyltryptamine, the prodrug of psilocin (4-OH-dimethyltryptamine) and the active constituent of magic mushrooms]; ayahuasca, a south American psychoactive brew, commonly prepared from the Banisteriopsis caapi vine, containing DMT (N,N-dimethyltryptamine) and alkaloid monoamine oxidase inhibitors; 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) and synthetic compounds such as lysergic acid diethylamide (LSD). Pharmacological effects of these agents are believed to emerge through activation of serotonin 5HT2A receptors and modulation of glutamatergic neurotransmission.Reference Madsen, Fisher and Burmester 93 , Reference Vollenweider and Preller 94 Imaging studies have shown that their potential clinical benefits may occur through altered activation of the default mode network and amygdala reactivity.Reference Carhart-Harris, Roseman and Bolstridge 95 Preclinical research shows that they enhance neuroplasticity through stimulation of hippocampal neurogenesis.Reference Morales-Garcia, Calleja-Conde and Lopez-Moreno 96
Psilocybin
Effects of psilocybin on depression and anxiety have been evaluated in three randomized, double-blind, placebo-controlled studies in patients with life-threatening cancer, included in a recent meta-analysis by Goldberg et al.Reference Goldberg, Pace and Nicholas 97 Psilocybin combined with behavioral interventions produced rapid and robust improvements in depressed mood and anxiety symptoms along with increases in quality of life in cancer patients. The anxiolytic and antidepressant effects of psilocybin were sustained in a large percentage of patients for at least 6 months post treatment. However, these results were limited to cancer patients experiencing depression and anxiety, which may not provide an accurate clinical representation of depression and anxiety observed in psychiatric patients. Nonetheless, these positive results encouraged further evaluation of psilocybin in other disorders. No serious adverse events were reported in any of these trials.
An open-label clinical study evaluated the effects of two doses of psilocybin (10 and 25 mg, 7 days apart) in 20 TRD patients.Reference Carhart-Harris, Bolstridge and Day 98 Patients received psychological support before, during, and after each session. Marked improvement in depressive symptoms, anxiety and anhedonia were detected after 1 week and 6 months post treatment. Psilocybin was well-tolerated by all TRD patients, and no serious or sustained adverse events occurred. Another clinical trial evaluated the effects of two oral doses of psilocybin in two sessions (session 1: 20 mg/70 kg; session 2: 30 mg/70 kg) given in the context of supportive psychotherapy in 27 adult MDD patients.Reference Davis, Barrett and May 99 Psilocybin treatment resulted in rapid and profound improvement in depressive symptoms as early as day 1 and effects were sustained for 4 weeks as assessed by GRID-Hamilton Depression Rating Scale (GRID-HAMD). 71% of patients treated with psilocybin showed greater than 50% reduction in depressive symptoms, and more than 50% of patients were in remission at 4 weeks. These findings further support the clinical benefits of psilocybin-assisted psychotherapy in improved treatment of MDD and TRD patients.
These two clinical trials, however, were confined to a small number of participants with limited racial diversity and lacked a placebo-controlled group. The most recent, randomized double-blind Phase II trial compared the efficacy of psilocybin with a well-known antidepressant, escitalopram, the first trial to make this direct comparison.Reference Carhart-Harris, Giribaldi and Watts 100 This was a larger trial with 59 MDD patients using functional magnetic resonance imaging (fMRI) and changes in depressive symptoms as assessed by the 16 item Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-16) as endpoints. In this unique trial design, effects of two 25 mg doses of psilocybin 3 weeks apart plus daily placebo were compared with two 1 mg doses of psilocybin 3 weeks apart plus daily escitalopram. At 6 weeks, there was no significant difference between the two groups in the primary outcome measure (QIDS-SR-16 change in score from baseline). Secondary outcome measures including remission at 6 weeks, and change from baseline score (at 6 weeks) on a range of other depression rating scales suggested that psilocybin treatment was superior to escitalopram, although the data were not corrected for multiple comparisons. No serious adverse events were observed in both groups.
Further trials are underway including the largest Phase II trial investigating the efficacy and safety of psilocybin therapy in 216 TRD patients enrolled in 21 sites across Europe and North America (ClinicalTrials.gov Identifier: NCT03775200). Another Phase II trial is evaluating the clinical efficacy of a single dose (25 mg) of psilocybin for MDD compared to a single dose of an active comparator (100 mg niacin) in 80 patients, assessed as the difference between groups in changes in depressive symptoms from baseline to Day 8 post-dose (ClinicalTrials.gov Identifier: NCT03866174). A large collaborative randomized double-blind placebo-controlled trial will assess the effects of oral psilocybin (5 and 25 mg) with psychological support compared with 100 mg nicotinamide (placebo) for TRD in 144 patients (ClinicalTrials.gov Identifier: NCT04670081). The outcomes of these trials should provide valuable insights into the mechanism of action of psilocybin in patients, and provide additional data on the efficacy and safety of psilocybin in severely depressed patients, which may support the potential regulatory approval of this unique therapy as a novel and improved treatment for MDD, TRD, and potentially, for patients with other neuropsychiatric disorders.
Ayahuasca and 5-MeO-DMT
An open-label trial evaluated the effects of an oral dose of Ayahuasca (2.2 mL/kg) in 17 patients with recurrent depression. Significant decreases in HAM-D, MADRS and Brief Psychiatric Rating Scales were detected as early as 80 minutes after ayahuasca treatment, and the antidepressant effects were sustained for 21 days. Single photon emission tomography detected increased cerebral blood flow in brain regions implicated in the regulation of mood and emotions.Reference Sanches, de Lima Osório and Dos Santos 101 Vomiting in 47% of participants was the only adverse event reported. An open-label randomized placebo-controlled trial assessed the antidepressant effects of a single dose of Ayahuasca (dose adjusted to contain 0.36 mg/kg of N,N-DMT) given over 8 hours and compared with placebo in 29 TRD patients. Ayahuasca treatment produced rapid and profound improvement in MADRS score compared to placebo as early as day 1 and the effects were sustained at day 7. More than one-third of patients went into remission,Reference Palhano-Fontes, Barreto and Onias 102 and drug treatment was generally well-tolerated in both studies, with transient anxiety, headache, and nausea and vomiting as the most common adverse events. An examination of group therapy in a naturalistic setting demonstrates beneficial effects of 5-MeO-DMT on anxiety and depression.Reference Davis, So and Lancelotta 103 This study surveyed 362 participants and found that 80% of respondents with depression or anxiety reported that their condition improved following the session with 5-MeO-DMT. Furthermore, these symptom improvements were associated with acute mystical effects of the drug. Additional randomized, double-blind, placebo-controlled trials are required to validate the efficacy, safety and tolerability of Ayahuasca and 5-MeO-DMT in larger numbers of patients with anxiety and depression.
Lysergic acid diethylamide
Earlier clinical studies reported that LSD-assisted psychotherapy resulted in dramatic improvement in symptoms of depression and anxiety, psychological isolation, fear of death and pain in advanced cancer patients.Reference Grof, Goodman, Richards and Kurland 104 A more recent double-blind, randomized, active placebo-controlled pilot study examined the effects of LSD-assisted psychotherapy in 12 patients with depression and anxiety associated with life-threatening diseases. Significant improvement in symptoms of anxiety were observed after 2 months of LSD treatment, and these effects were sustained for 12 months.Reference Gasser, Holstein and Michel 105 These results indicate that LSD-assisted psychotherapy can reduce anxiety, suggesting that larger controlled studies in patients with anxiety and depression are needed.Reference Fuentes, Fonseca and Elices 106
Safety and tolerability
Different psychedelic agents were generally well-tolerated in different clinical trials. The most common adverse effects were transient anxiety, headaches, nausea and small increases in heart rate and blood pressure.Reference Muttoni, Ardissino and John 107 Psychedelics have proven remarkably safe with low risk of addiction when used in a clinical setting where the paradigm is treatment with one or two high doses preceded and followed by extensive psychotherapy. 90 This integration is essential to enable the patient to make sense of the experience, and to gain maximum and long-lasting benefits. Significant investment in psychotherapy in psychedelic trials may limit affordability, although with such long-lasting beneficial effects in severely depressed patients, the economic and health care benefits are likely to be significant.
Summary and Conclusions
The medical community and the pharmaceutical industry have recognized the importance and the need to develop novel antidepressant drugs that deviate from the “me too” approach, and extend beyond the traditional monoamine targets. Attention has shifted towards other brain neurotransmitters and pathways, including the glutamatergic and GABAergic systems, which now constitute attractive targets for developing novel antidepressants with novel mechanisms of action. The earlier clinical studies with ketamine, and the rapid and profound improvement of depressive symptoms in TRD patients after IV administration of ketamine ushered a new era of antidepressant drug development and encouraged the development of novel glutamatergic agents along the same mechanistic lines. The recent FDA approval of intranasal Esketamine in conjunction with an oral antidepressant for the treatment of adult TRD patients marks a new milestone in approving new antidepressants with novel mechanisms of actions. Several other glutamatergic modulating agents, including AXS-05, REL-1017, AV-101, SLS-002, AGN241751, and PCN-101, are in different stages of clinical development for MDD and TRD.
The failure of rapastinel, an allosteric modulator of the glycine site of the NMDA receptor complex, in several Phase III clinical trials, in spite of its supportive preclinical profile and encouraging Phase II results, highlights the difficulties in achieving sustained success in developing glutamatergic agents with superior efficacy for the improved treatment of MDD and TRD. This failure also minimizes the translational value of preclinical models of depression, and even early stages of clinical development in accurately predicting the clinical outcomes of the more pivotal Phase III clinical trials. Nonetheless, extensive preclinical assessment of novel compounds remains as an essential step in drug development prior to initiating more intensive and expensive clinical trials.
Targeting the GABAergic system resulted in the development of brexanolone, the first FDA-approved drug for treatment of PPD in adults. Other GABAergic modulating agents (zuranolone and ganaxolone) are now in different stages of clinical development for MDD, TRD, and other indications such as postpartum depression (PPD) and treatment of seizures in CDKL5 deficiency disorder. The emergence of psychedelic drugs and the potentially promising clinical results of psilocybin in the treatment of TRD patientsReference Davis, Barrett and May 99 have generated interest in this class of drugs as novel groundbreaking therapies for depression and other psychiatric disorders and have garnered the attention of clinical experts and business investors despite the limited patent protection of these agents. Nonetheless, the approval of these agents remains challenging because of their current Drug Enforcement Administration (DEA) scheduling status, and the possibility that the FDA may place restrictions on their use, which may minimize their utility in clinical practice.
Depression is considered the second leading cause of disability worldwide, and is associated with substantial socio economic burden that stems from the greater use of healthcare resources and reduced work productivity.Reference Egede, Bishu, Walker and Dismuke 108 It is known that the costs of developing new drugs for depression from the laboratory bench to the patient’s bed are exuberant. However, the new drugs in development should not be priced at a premium that makes them beyond the reach of many MDD and TRD patients. A recent study suggested the current price of the newly approved antidepressant esketamine of approximately $240 per dose is not cost-effective, and the drug is unlikely to be cost-effective unless its price falls by more than 40%.Reference Ross and Soeteman 109 In addition, the high cost of a single course of treatment with brexanolone of $34,000 excluding hospitalization costs marks a big barrier to many PPD patients who may benefit from the treatment.Reference Shukla, Jhai and Sadasivam 110 These studies highlight the importance of conducting pharmacoeconomic analysis of the novel antidepressant drugs in the pipeline to ensure their prices provide cost-benefit and cost-effective advantages, which will make them easier to be covered by medical insurance and healthcare payors, and more accessible to MDD and TRD patients.
Author Contributions
Conceptualization: X.G., P.D., J.C.N., F.I.T.; Investigation: X.G., P.D., J.C.N., F.I.T.; Validation: P.D., J.C.N., F.I.T.; Visualization: X.G., P.D., J.C.N., F.I.T.; Writing—original draft: X.G., P.D., J.C.N., F.I.T.; Writing—review and editing: X.G., P.D., J.C.N., F.I.T.
Disclosures
Joanna C. Neill is a scientific advisor to Beckley Psytech and Albert Labs. Xenia Gonda, Peter Dome, and Frank I. Tarazi reported no conflicts of interest.



