Introduction
A complex syndrome of bizarre motor behavior, impaired volition, and vegetative abnormalities was described by Karl Kahlbaum in the 1870s and termed catatonia.Reference Kahlbaum 1 Numerous inconsistent descriptions were to follow in the psychiatric literature.Reference Fink and Taylor 2 – Reference Walther and Strik 5 The debate included the nosology of the syndrome, its operationalization, and the suspected pathology. Even its extinction by current antipsychotic treatment has been suggested.Reference Stompe, Ortwein-Swoboda, Ritter, Schanda and Friedmann 6 Once detected, catatonia may be effectively treated in many patients.Reference Fink, Shorter and Taylor 7 , Reference Sienaert, Dhossche, Vancampfort, De Hert and Gazdag 8 Strikingly, most scientific articles on catatonia are merely case reports or case series, but original articles are much less available. Recently, progress has been achieved by studies reporting that catatonia is still highly prevalentReference Peralta, Campos, de Jalon and Cuesta 9 – Reference Kruger, Bagby, Hoffler and Braunig 12 and not restricted to schizophrenia spectrum disorders.Reference Kruger, Bagby, Hoffler and Braunig 12 – Reference Dhossche and Wachtel 14 Finally, catatonia has been separated from schizophrenia in Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).Reference Tandon, Heckers and Bustillo 15 Here, we will summarize the literature of the past decade focusing on prevalence rates and classification issues. We will also discuss putative pathobiology and propose a new research strategy to approach catatonia.
The Catatonic Syndrome
The rich clinical descriptions of catatonia are summarized elsewhere.Reference Heckers, Tandon and Bustillo 3 – Reference Walther and Strik 5 , Reference Fink, Shorter and Taylor 7 , Reference Fink 16 Up to 40 different signs and symptoms have been associated with catatonia.Reference Ungvari, Caroff and Gerevich 4 , Reference Fink 16 These signs may be summarized in 4 groupsReference Walther and Strik 5 : pure motor signs (eg, posturing, rigor, immobility), disturbances of volition (eg, ambitendence, negativism, automatic obedience), inability to suppress complex motor activities (eg, stereotypies, rituals, echophenomena), and autonomic instability (eg, tachycardia, hyperthermia). Symptoms usually wax and wane sometimes within 1 hour. Even though some are more prevalent than others, there is no single specific symptom to identify catatonia. Some authors, relying heavily on Kahlbaum’s initial descriptions,Reference Fink 16 , Reference Northoff, Koch and Wenke 17 also consider affective disturbances and behavioral problems (eg, nudism) as part of the catatonic syndrome. Finally, the catatonic syndrome may become malignant with increased mortality,Reference Fink and Taylor 2 , Reference Fink 16 particularly when autonomic instability is included.
The catatonia syndrome frequently occurs in schizophrenia spectrum disorders and affective disorders, but also in autism, dementia, intoxications, and in general medical conditions. The onset and duration of symptoms vary considerably. Particularly among chronic schizophrenia patients, cases with chronic catatonia course have been reported.Reference Ungvari, Leung, Ng, Cheung and Leung 11 Some catatonia patients experience complete remission within 24 hours.Reference Lin and Huang 18 Acute and chronic forms of catatonia share the same symptoms, but some clinical differences in symptom endorsement frequencies have been noted, and benzodiazepines are less effective in chronic catatonia.Reference Lin and Huang 18 – Reference Ungvari, Leung, Wong and Lau 21 Catatonia may occur in children. As in adults, insidious onset is associated with poorer outcome.Reference Raffin, Zugaj-Bensaou and Bodeau 22
Classification Issues: DSM-5 vs. ICD-10
DSM-5 and the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) differ substantially in their definitions and classifications of catatonia (see Table 1). While DSM-5 conceptualized catatonia as a widely independent syndrome, ICD-10 allows diagnosing catatonia only in the context of schizophrenia or as a syndrome due to an organic brain disorder. This might be due to the perception of catatonia in the early 1990s when ICD-10 was proposed. DSM-IV, however, which was published a few years before ICD-10, already offered the opportunity to code catatonia as a specifier of major mood disorders.Reference Tandon, Heckers and Bustillo 15 In DSM-5, catatonia is now recognized in all psychotic and major mood disorders as a syndrome due to general medical conditions, or as a syndrome not otherwise specified; this allows coding catatonia in the context of other psychiatric disorders, such as autism or obsessive compulsive disorder.
Table 1 Diagnostic criteria of catatonia
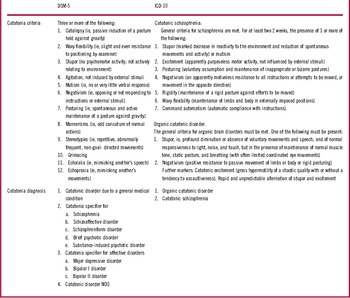
Besides the nosologic restrictions, the systems differ in the diagnostic criteria of catatonia (see Table 1). ICD-10 lists only 2 symptoms for organic catatonic disorder (stupor and negativism) and 7 symptoms for the catatonic subtype of schizophrenia. In contrast, DSM-5 lists 12 symptoms for catatonia independent of the underlying disorder. There is considerable overlap between the lists, as 5 out of 7 ICD-10 criteria are also found in the DSM-5 criteria (posturing, negativism, stupor, waxy flexibility, and excitement/agitation). Yet, rigor and automatic obedience are not included in the DSM-5 criteria, while ICD-10 lacks relevant items such as the echophenomena, mutism, mannerisms, stereotypies, and grimacing. The diagnostic thresholds are different and affect the incidence of catatonia in schizophrenia.Reference Stuivenga and Morrens 23 , Reference Wilson, Niu, Nicolson, Levine and Heckers 24 Three or more items without time limit are required in DSM-5, while only 1 lasting for 2 weeks is sufficient in ICD-10.
The ICD-10 catatonia concept has 2 major limitations: persistent catatonia will eventually be labeled as schizophrenia, and catatonia observed in the context of neurodevelopmental disorders or nonschizophrenic psychoses cannot be coded. Instead, major advances of DSM-5 over DSM-IV are the introduction of a catatonia specifier for psychotic disorders (see Table 1), the change of the diagnostic criteria (3 or more of 12 signs), the introduction of “catatonia not otherwise specified (NOS),” and the waiving of the catatonic subtype of schizophrenia.Reference Tandon, Heckers and Bustillo 15 The DSM-5 catatonia specifier has been warmly greeted by catatonia experts, although its impact on treatment is unclear: As Max Fink pointed out, there might be problems when treating the catatonia syndrome and the underlying disorder concurrently.Reference Fink 16 Still, many patients will have their underlying condition when catatonia has already been relieved.Reference Tandon, Heckers and Bustillo 15
Clinical Presentation
Prevalence
Prevalence rates of catatonia vary depending on catatonia concepts and criteria (see Table 2). In schizophrenia, catatonia can occur irrespective of disease state (first episode or chronic course) and treatment status (never medicated or medicated).Reference Walther and Strik 5 , Reference Peralta, Campos, de Jalon and Cuesta 9 , Reference Ungvari, Leung, Ng, Cheung and Leung 11 Prevalence rates increase when catatonia rating scales such as the Bush Francis Catatonia Rating Scale (BFCRS)Reference Bush, Fink, Petrides, Dowling and Francis 25 are applied. DMS-5 or ICD-10 criteria are more conservative. In mixed inpatient populations of psychiatric institutions, catatonia appears to have a prevalence of 10–25%.Reference Stuivenga and Morrens 23 , Reference Grover, Chakrabarti, Ghormode, Agarwal, Sharma and Avasthi 26 As noted by several authors, the use of clinical rating scales or experts is superior in detecting catatonia compared to registers of clinical diagnoses.Reference Stompe, Ortwein-Swoboda, Ritter, Schanda and Friedmann 6 , Reference Peralta, Campos, de Jalon and Cuesta 9 , Reference van der Heijden, Tuinier, Arts, Hoogendoorn, Kahn and Verhoeven 10 , Reference Stuivenga and Morrens 23 , Reference Peralta and Cuesta 27 , Reference Kleinhaus, Harlap and Perrin 28 Reports agree that the presence of 3 or more catatonia signs have optimal sensitivity and specificity to detect catatonia among psychotic patients.Reference Peralta, Campos, de Jalon and Cuesta 9 , Reference Grover, Chakrabarti, Ghormode, Agarwal, Sharma and Avasthi 26 , Reference Peralta and Cuesta 29
Table 2 Prevalence rates of catatonia

Reported catatonia prevalence rates in mixed patient groups were quite similar between affective disorders and psychotic disorders.Reference Stuivenga and Morrens 23 , Reference Grover, Chakrabarti, Ghormode, Agarwal, Sharma and Avasthi 26 However, differences in onset, number of signs, and course were noted between catatonia due to schizophrenia and so-called idiopathic catatonia, ie, without any underlying axis-I disorder.Reference Krishna, Maniar and Harbishettar 30 Likewise, symptom presentation slightly differs between catatonia due to schizophrenia and affective disorders.Reference Kruger, Bagby, Hoffler and Braunig 12 , Reference Stuivenga and Morrens 23 , Reference Worku and Fekadu 31
Even though a few studies on the prevalence of catatonia in mixed patient groups are available,Reference van der Heijden, Tuinier, Arts, Hoogendoorn, Kahn and Verhoeven 10 , Reference Stuivenga and Morrens 23 , Reference Wilson, Niu, Nicolson, Levine and Heckers 24 , Reference Grover, Chakrabarti, Ghormode, Agarwal, Sharma and Avasthi 26 there is a lack of systematic investigations on the presentation of catatonia in different patient groups. While some studies in psychotic disorders assessed catatonia among other motor abnormalities,Reference Peralta and Cuesta 27 , Reference Peralta, Campos, De Jalon and Cuesta 32 – Reference Compton, Fantes, Wan, Johnson and Walker 34 most reports fail to delineate catatonia from other movement disorders. Finally, studies that focus on the duration and course of catatonia are needed.
Factor structure
As catatonia includes a variety of symptoms, different forms have been proposed. Many clinicians follow the classical distinction between retarded and excited catatonia.Reference Fink 16 Depending on the instruments used and the sample investigated, studies reported 3,Reference Peralta, Campos, de Jalon and Cuesta 9 , Reference Wilson, Niu, Nicolson, Levine and Heckers 24 4,Reference Kruger, Bagby, Hoffler and Braunig 12 , Reference Ungvari, Goggins, Leung and Gerevich 35 or 6Reference Stuivenga and Morrens 23 independent factors. Consistently, studies disentangle excited catatonia, retarded catatonia, and 1 factor describing disturbances of volition. Besides the need to further develop appropriate clinical instruments to cover the catatonia syndrome among various underlying conditions, the precise structure of catatonia has yet to be discovered.Reference Wilson, Niu, Nicolson, Levine and Heckers 24
Special forms
As acknowledged by DSM-5, catatonia may occur in various conditions across the lifespan. In childhood onset schizophrenia with catatonic features, comorbid medical conditions are found in 22% and developmental disorders in 31% of cases.Reference Consoli, Raffin and Laurent 36 Childhood catatonia often presents as excited catatonia including aggression.Reference Ghaziuddin, Dhossche and Marcotte 37 Even though, in many cases, catatonia is associated with another psychiatric disorder or general medical condition, in some cases catatonia is the only detectable syndrome. Based on their impressive clinical data, Peralta et al Reference Peralta, Campos, De Jalon and Cuesta 32 proposed to separate idiopathic catatonia from catatonia secondary to a psychiatric disorder or medical condition.
In non-affective psychoses, catatonia frequently co-occurs with other motor abnormalities, particularly abnormal involuntary movements and parkinsonism.Reference Peralta and Cuesta 27 , Reference Peralta, Campos, De Jalon and Cuesta 32 , Reference Compton, Fantes, Wan, Johnson and Walker 34 , Reference Docx, Morrens and Bervoets 38 Besides the co-existence of various motor abnormalities, there is conceptual overlap, as some symptoms such as rigor are classified either as a sign of parkinsonism or of catatonia.Reference Walther and Strik 5 In schizophrenia, catatonia symptoms interfere with correct performance of hand gestures and even with nonverbal social perception.Reference Walther, Vanbellingen, Muri, Strik and Bohlhalter 39 , Reference Walther, Stegmayer and Sulzbacher 40 Thus, catatonia may strongly hamper nonverbal communication and contribute to poor social functioning in schizophrenia.
Treatment
Evidence from numerous case reports and a few controlled clinical studies advocates the use of benzodiazepines in adultsReference Sienaert, Dhossche, Vancampfort, De Hert and Gazdag 8 , Reference Fink 16 and in children.Reference Raffin, Zugaj-Bensaou and Bodeau 22 Diazepam, clonazepam, or oxazepam are also effective.Reference Sienaert, Dhossche, Vancampfort, De Hert and Gazdag 8 In chronic schizophrenia, the situation is less clear.Reference Ungvari, Chiu, Chow, Lau and Tang 20 In subjects with insufficient response to benzodiazepines or in life-threatening conditions, electroconvulsive therapy (ECT) is the method of choice to treat catatonia. Patients with chronic catatonia seem to benefit from a combination of ECT and clozapine.Reference Sienaert, Dhossche, Vancampfort, De Hert and Gazdag 8
In order to treat the underlying condition, patients with catatonia often receive antipsychotic drugs when catatonia accompanies schizophrenia. The use of antipsychotic agents in catatonia is intensely debated.Reference Sienaert, Dhossche, Vancampfort, De Hert and Gazdag 8 , Reference Fink 16 An excellent study investigated the effect of a 4-week trial of antipsychotics on motor syndromes in 100 medication-naïve, first-episode psychosis patients.Reference Peralta and Cuesta 33 Catatonia was treatment responsive in 15/18 (83%) cases, remained unchanged in 3 subjects, and appeared with treatment in 2 patients.
Suspected Pathophysiology
Neurotransmitter systems
Several neurotransmitter disturbances have been discussed as putative causes for catatonia, including dopamine, glutamate, and gamma-aminobutyric acid (GABA). Dopamine has been put forward as dopamine antagonism may produce rigor and immobility, and dopamine-D2 antagonists may worsen catatonia in some cases.Reference Sienaert, Dhossche, Vancampfort, De Hert and Gazdag 8 However other signs of catatonia cannot be explained by dopamine antagonist action. One syndrome closely related to catatonia—the neuroleptic malignant syndrome—is probably caused by antidopaminergic drugs.Reference Fink 16 Dopamine agonists, however, fail to alleviate the neuroleptic malignant syndromeReference Sienaert, Dhossche, Vancampfort, De Hert and Gazdag 8 as well as chronic catatonia.Reference Ungvari 41 The few studies on dopamine metabolism or receptor occupancy in catatonia remain inconclusive.Reference Lauer, Schirrmeister and Gerhard 42 , Reference Northoff 43 Glutamate dysfunction may contribute to some of the catatonia phenotypes. The N-methyl-D-aspartate (NMDA)-receptor antagonist ketamine elicited catatonia-like signs when administered in healthy subjects.Reference Gouzoulis-Mayfrank, Heekeren and Neukirch 44 In line with this, a mouse model of reduced NMDA-receptor expression indicated abnormal motor and social behavior with face validity when compared to catatonia.Reference Mohn, Gainetdinov, Caron and Koller 45 Furthermore, the clinical presentation of many subjects with anti-NMDA-receptor encephalitis mimics acute catatonia with stereotypies, mutism, echophenomena, rigidity, and abnormal involuntary facial movements.Reference Dalmau, Lancaster, Martinez-Hernandez, Rosenfeld and Balice-Gordon 46 In fact, some acute catatonia cases may even resemble misdiagnosed anti-NMDA-receptor encephalitis.Reference Steiner, Walter and Glanz 47 In contrast, anecdotal reports suggest some efficacy of the NMDA-antagonist amantadine in treating catatonia.Reference Sienaert, Dhossche, Vancampfort, De Hert and Gazdag 8 , Reference Northoff 43 GABAergic drugs are most effective in treating acute catatonia, as evidenced by numerous case reports and clinical trials.Reference Sienaert, Dhossche, Vancampfort, De Hert and Gazdag 8 , Reference Northoff 43 Moreover, decreased GABAA-receptor density was detected in the left sensorimotor cortex of catatonia patients, which correlated with the severity of catatonia.Reference Northoff, Steinke and Czcervenka 48 Very recently, antibodies against GABAA-receptor subunits were detected in patients with neuropsychiatric syndromes, including 2 subjects with catatonia-like behaviors; 1 of the 2 improved rapidly following plasma exchange.Reference Pettingill, Kramer and Coebergh 49 Still, a controlled clinical trial of GABA agonist lorazepam indicated no effect on chronic catatonia.Reference Ungvari, Chiu, Chow, Lau and Tang 20 Taken together, alterations in GABAergic and glutamatergic neural activity may contribute to some but not all phenotypes of catatonia, while the case is much less clear for dopamine.
Brain circuitry
The neuroimaging literature on catatonia still is slowly evolving.Reference Walther 50 To many patients with catatonia, the scanning procedure would be intolerable, while others are incapable of providing informed consent. The current literature is limited to case series on cerebral metabolism or regional cerebral blood flow obtained during the resting state. Very few studies have managed to test neural activation during tasks. Therefore, neuroimaging studies in catatonia are particularly affected by selection bias, strongly limiting the generalizability of results.
Alterations of brain function or structure due to catatonia are found within the cerebral motor circuit.Reference Walther 50 The majority of studies reported hypoactivity in cortical motor areas of the frontal and parietal cortex. Early work on regional cerebral blood flow (rCBF) indicated frontal and parietal hypoperfusion in mixed groups of predominantly akinetic catatonia.Reference Northoff, Steinke and Nagel 51 – Reference Satoh, Suzuki and Narita 53 Furthermore, some reports noted an increase of frontal motor and parietal rCBF during the improvement of catatonia by ECT.Reference Galynker, Weiss, Ongseng and Finestone 52 , Reference Escobar, Rios and Montoya 54 Moreover, akinetic catatonia patients had delayed onset of movement-related potentials and readiness potentials in motor areas, which correlated with catatonia severity.Reference Northoff, Pfennig and Krug 55 Likewise, few studies consistently found reduced neural activation in cortical motor and premotor areas, as well as in the parietal cortex in catatonia during finger-tapping or finger-opposition tasks.Reference Northoff, Braus and Sartorius 56 – Reference Scheuerecker, Ufer and Kapernick 58 Finally, 2 studies suggested impaired orbitofrontal function during processing of negative emotions in catatonia.Reference Northoff, Kotter and Baumgart 59 , Reference Richter, Grimm and Northoff 60
In contrast to the above mentioned studies, there have been reports of chronic catatonia patients in whom cerebral correlates of catatonia symptoms revealed different patterns. Three cases of chronic catatonia presented with separate patterns of brain metabolism according to symptoms: One patient experienced frontal hypermetabolism and thalamic hypometabolism while presenting with speech prompt symptoms, ie, immediate verbal response. In contrast, 2 subjects with speech sluggish catatonia presented the opposite pattern: left frontal hypometabolism and thalamic hyperactivity.Reference Lauer, Schirrmeister and Gerhard 42 Likewise, a case of very late onset catatonia was reported to have retarded catatonia and cerebral hypoperfusion within striatum and thalamus, but hyperperfusion in the left lateral frontal cortex; both localized perfusion changes were ameliorated by effective therapy.Reference Tsujino, Nemoto and Yamaguchi 61 The identical pattern of cerebral metabolism was reported in a case of a young girl with catatonia with stereotypies and mutism/immobility.Reference De Tiege, Bier and Massat 62 Near Infrared spectroscopy revealed phasic hyperactivity in the left anterior prefrontal cortex during episodes of staring, mutism, and catalepsy in a patient with treatment-resistant catatonia.Reference Grignon, Forget, Durand and Huppert 63 Thus, patterns of altered cerebral metabolism or neural activity are not necessarily driven by the presence or absence of catatonia, but may correspond to specific symptoms. For further illustration, please also see our Case Report (in the Appendix) of a young patient with chronic catatonia who experiences mainly volitional problems, eg, during movement initiation while the movements are executed rapidly and correctly once started. In contrast to the findings in akinetic catatonia, his perfusion MRI indicated abnormal hyperperfusion at rest within the supplementary motor area (see the Case Report in the Appendix and Figure 1). We have introduced a rating scale for 3 psychopathological dimensions of psychoses, the Bern Psychopathology Scale,Reference Strik, Wopfner and Horn 64 including a motor dimension that separates motor inhibition from excitement. In schizophrenia, inhibition in this motor domain was associated with increased volume of the supplementary motor area.Reference Stegmayer, Horn and Federspiel 65
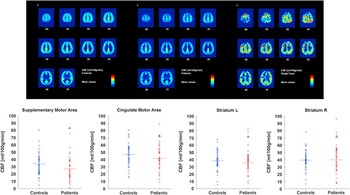
Figure 1 Cerebral resting state perfusion in healthy controls (upper row left), schizophrenia patients (upper row middle), and a single catatonia patient with predominantly impaired volition and motor initiation (upper row right). Numbers below the slices indicate the z coordinate of the axial slice. Perfusion values of 4 motor regions are given at the bottom (controls in blue, patients in red, catatonia case indicated by black triangle).
While some progress was made in unraveling the pathobiology of catatonia in the 1990s and 2000s, we are currently facing a deadlock of research on this topic. In our view, there are 2 main reasons for this dilemma: the clinical description of the syndrome and the lack of sufficient neuroscientific methods. While DSM-5 has now offered a way to detect and classify catatonia, in many instances providing a clear general set of criteria, a number of problems remain: the heterogeneity of signs in the clinical presentation, the overlap of signs with other syndromes such as parkinsonism, the association with different underlying disorders, and the heterogeneous course. To date, it is unclear whether the catatonia syndrome resembles a general clinical phenotype with different underlying pathomechanisms or whether catatonia has a common phenotype and pathobiology. Currently, the first assumption seems more likely. Several psychiatric and medical conditions may produce transient catatonia and a few chronic forms of catatonia. The majority responds well to rather unspecific treatments, such as benzodiazepines and ECT.Reference Sienaert, Dhossche, Vancampfort, De Hert and Gazdag 8 The association with a broad variety of underlying conditions that have not very much in common argues against a common cause for catatonia. Given this magnitude of heterogeneity in symptom presentation, course, and underlying condition, we may not be able to find a substrate when applying neuroimaging, endocrine markers, or immunological assays in a group of catatonia patients.
Proposed research strategy
The changes in DSM-5 will hopefully increase catatonia awareness. Progress in catatonia research may be achieved at a basic clinical level and at a neuroscientific level. The basic clinical level would include data on frequencies and enhanced catatonia instruments. With the new DSM-5 criteria, there is clearly a need for more prevalence data, which must also take into account the heterogeneous course of catatonia. In addition, as pointed out by several groups, the current rating scales require well-considered improvements, as the structure of catatonia has not yet been fully discovered.Reference Wilson, Niu, Nicolson, Levine and Heckers 24 On the neuroscientific level, which must rely on clear clinical descriptions and delineations, there is a need for further neuroimaging studies. In fact, most neuroimaging studies would not meet the current standards of image processing and data reporting. Advances in structural neuroimaging and new methods of assessing the brain’s resting state could contribute to the understanding of the catatonia neurobiology. Maybe these techniques could disentangle different types of disturbances within the motor circuitry. Finally, we may go on to test specific noninvasive brain stimulation techniques in catatonia. Particularly, as the pathobiology is very likely to be heterogeneous, individualized noninvasive brain stimulation could become a safe and powerful tool.
Conclusion
Catatonia is a severe psychomotor syndrome associated with various psychiatric disorders and medical conditions. It responds well to benzodiazepines or ECT. Classification and prevalence rates differ according to the diagnostic systems. The pathophysiology is still widely unknown. The new diagnostic criteria will hopefully encourage more clinical and basic research on catatonia with sufficient methods.
Disclosures
Sebastian Walther has the following disclosures: Janssen Cilag, educational speaker, speaker’s fee; Eli Lilly, educational speaker, speaker’s fee; Sandoz, educational speaker, speaker’s fee; Lundbeck and Otsuka, advisory board member, honoraria. Werner Strik does not have anything to disclose.
Appendix: Case Report
An 18-year-old patient had been suffering from chronic catatonia with predominant negativism, blocking, staring, posturing, rituals, and stereotypy for more than 2 years. He experienced an insidious onset that first included generalized slowing. For most of this period, severity remained basically the same, but symptom intensity waxed and waned. Little benefit was achieved by administering clozapine 200 mg/d. On the Bush Francis Catatonia Rating Scale, he had a score of 19. Interestingly, he may exhibit good performance and endurance in physical exercise once the movements are started, particularly when triggered by external stimuli. Thus, the main clinical problem is volition and movement initiation. Perfusion MRI with arterial spin labeling (ASL) indicated increased resting state cerebral blood flow predominantly in premotor areas of the brain (see Figure 1, top panel). In comparison to healthy controls and a cohort of schizophrenia patients, he had maximum perfusion in the supplementary motor area and high perfusion in the cingulate motor area, but average values in the bilateral striatum (see Figure 1, bottom panel). Thus, the problem with this patient appears to be one of ineffective motor planning, which seems to be related to hyperperfused premotor areas.





