Clay minerals and iron oxide minerals are common solid-phase components in soils. Clay minerals (including kaolinite, montmorillonite, illite, sepiolite, etc.) are composed mainly of hydrated magnesium–aluminium silicates (Uddin, Reference Uddin2017; Guo et al., Reference Guo, Liu, Gates and Zhou2020; Zhou et al., Reference Zhou, Niu, Liu, Chen, Li and Gates2022). Iron oxides found in soils include goethite, ferrihydrite and hematite, among others (Ma et al., Reference Ma, Lei, Weng, Li, Chen and Islam2019; Lai et al., Reference Lai, He, Zhou, Huang, Yao and Lai2021). Soil minerals constitute an important medium for the accumulation of heavy-metal ions on account of their large specific surface area and abundant surface-adsorption sites, leading to favourable adsorption capacities for lead (Pb2+), cadmium (Cd2+), zinc (Zn2+) and other metal ions (Bedelean et al., Reference Bedelean, Măicăneanu, Burcă and Stanca2009; Mbaye et al., Reference Mbaye, Diop, Miehe-Brendle, Senocq and Maury2014; Vhahangwele & Mugera, Reference Vhahangwele and Mugera2015; Yin et al., Reference Yin, Tan, Liu, Wang, Liang and Qu2016; Lin et al., Reference Lin, Sun, Su, Owens and Chen2019; Otunola & Ololade, Reference Otunola and Ololade2020).
Lead, one of the common ‘five toxic elements’ in ecosystems, is non-biodegradable, accumulable and biotoxic (Bourliva et al., Reference Bourliva, Michailidis, Sikalidis, Filippidis and Betsiou2013; Rui et al., Reference Rui, Wu, Ji, Liu, Wang and Ito2019; Ramola et al., Reference Ramola, Belwal, Li, Wang, Lu and Yang2020). The adsorption characteristics and mechanisms of Pb2+ on various soil minerals vary significantly. Zhang & Hou (2008) demonstrated that the adsorption mechanism of Pb2+ on montmorillonite could be ascribed chiefly to the chemical binding of Pb2+ to surface hydroxyl groups and electrostatic binding. Tang et al. (Reference Tang, Tang, Li, Chen, Kou and Sun2009) observed that the adsorption of Pb2+ on kaolinite occurred through ion exchange and complexation. Trivedi et al. (Reference Trivedi, Dyer and Sparks2003) proposed that Pb2+ was adsorbed on ferrihydrite mostly through inner-sphere complexation. The adsorption mechanism determines the occurrence state of Pb2+ in soils, which in turn affects the fate of Pb2+. However, quantification of the adsorption mechanisms of Pb2+ by soil minerals (e.g. montmorillonite, goethite, ferrihydrite, kaolinite) has been determined only rarely. It has been reported that stepwise extraction could quantify the contributions of adsorption mechanisms, improving understanding of the occurrence state and stability of metal ions. For instance, Shen et al. (Reference Shen, Zhang, Jin, McMillan and Al-Tabbaa2017) quantified the contributions of exchangeable state (1.38–4.29%), precipitation (75.61–85.76%) and complexation (10.40–22.86%) adsorption mechanisms for Pb2+ adsorbance on three biochars. Cao et al. (Reference Cao, Xiao, Shen, Ji, Zhang and Gao2019) quantified the adsorption mechanism of Pb2+ on wheat straw biochar, in which the contributions of major adsorption mechanisms such as ion exchange and precipitation were 74.79% and 21.92%. These studies quantifying adsorption mechanisms through stepwise extraction have focused on biochars. However, current research on the mechanism of Pb2+ adsorption by montmorillonite, goethite, ferrihydrite and kaolinite has focused mostly on qualitative analysis, while quantitative analysis of this topic has been limited. Therefore, the quantification of the adsorption mechanisms of Pb2+ by montmorillonite, goethite, ferrihydrite and kaolinite based on stepwise extraction has important theoretical significance for our in-depth understanding of the occurrence state of Pb2+ in soils.
In the present work, the Pb2+ adsorption properties and mechanisms by montmorillonite, goethite, ferrihydrite and kaolinite were investigated using batch adsorption tests and characterization techniques. The contributions of various adsorption mechanisms were quantified using a stepwise extraction method to provide the theoretical foundations for an in-depth understanding of the occurrence state of Pb2+ in soils.
Materials and methods
Chemicals and materials
Iron nitrate nonahydrate (Fe(NO3)3⋅9H2O) was kindly provided by the Zhiyuan Chemical Reagent Co., Ltd (Tianjin, China). Analytical reagents of lead nitrate (Pb(NO3)2), nitric acid (HNO3), sodium hydroxide (NaOH) and potassium hydroxide (KOH) were manufactured by Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
The kaolinite was collected from Yangshan, Suzhou, China, and the X-ray fluorescence (XRF) results showed that the kaolinite was composed mainly of SiO2 (49.41 wt.%), Al2O3 (46.35 wt.%), Fe2O3 (1.14 wt.%) and others (3.10 wt.%). The montmorillonite was collected from Chifeng City Wuhuatianbao Mineral Materials Co., Ltd (Inner Mongolia, China). According to our previous work, the montmorillonite was composed of SiO2 (69.46 wt.%), Al2O3 (17.30 wt.%), Fe2O3 (6.77 wt.%), MgO (2.75 wt.%), CaO (1.97 wt.%) and others (1.75 wt.%) based on the XRF results (Zhang et al., Reference Zhang, Zou, Liu, Chen, Dong and Ji2022). The preparation of goethite was done according to the process described by Notini et al. (Reference Notini, Latta, Neumann, Pearce, Sassi and N Diaye2018) and synthesized via the hydrothermal method of mixing Fe(NO3)3⋅9H2O and KOH in an alkaline environment. Ferrihydrite was prepared according to the method of Schwertmann & Cornell (Reference Schwertmann and Cornell2008), where Fe(NO3)3⋅9H2O was dissolved and then the pH of the solution was adjusted to 7–8 with NaOH, allowing Fe3+ hydrolysis to generate ferrihydrite. All samples were ground and passed through a 0.075 mm sieve and then placed in a desiccator.
Characterization
The composition and crystal structure of the four minerals before and after Pb2+ adsorption were examined using X-ray diffraction (XRD; DX-2700, Dandong, China) at a scanning angle of 5–70°2θ. The morphologies and elemental distributions of the four minerals with and without Pb2+ adsorption were examined using scanning electron microscopy (SEM; Hitachi SU8020; Hitachi, Tokyo, Japan). The functional groups of the four minerals with and without adsorption of Pb2+ were recorded using a Fourier-transform infrared (FTIR) spectrometer (VERTEX 70; Bruker, Germany) in the wavelength range of 400–4000 cm–1. The ζ-potential measurements of the four minerals were conducted at pH 1–12 using a Zetasizer Nano-ZS90 (Malvern Instruments, Inc., Malvern, UK). The isoelectric points of the four minerals were determined from their ζ-potentials and pH values (Fang et al., Reference Fang, Chen, Lin and Guan2014). The distribution of Pb2+ species in the pH range of 1–12 was simulated using Visual MINTEQ 3.1.
Batch adsorption experiments
The effects of initial concentration (5, 10, 20, 50, 80, 100 mg L–1) and reaction time (5, 10, 20, 40, 60, 120, 180, 240 min) on the adsorption behaviour of Pb2+ by montmorillonite, goethite, ferrihydrite and kaolinite were studied using batch experiments. All Pb2+ solutions were obtained by diluting 10 g L–1 Pb(NO3)2. The 0.5 g L–1 adsorbent was added into a polypropylene centrifuge tube containing Pb2+ solutions at various pH values (4, 5 or 6). The mixture was shaken continuously for a certain period of time (ranging between 5 and 240 min). Then, the concentration of Pb2+ was measured using a flame atomic adsorption spectrophotometer (wys-2200, Wayeal, China).
Stepwise extraction of adsorbed Pb2+
In this study, by following the approaches of Shen et al. (Reference Shen, Zhang, Jin, McMillan and Al-Tabbaa2017) and Tessier et al. (Reference Tessier, Campbell and Bisson1979), the adsorption capacities of montmorillonite, goethite, ferrihydrite and kaolinite for Pb2+ and the contributions of various adsorption mechanisms were analysed. The adsorption mechanisms of Pb2+ included chiefly physisorption (Q phy), ion exchange (Q exc), precipitation (Q pre), electrostatic (Q ele) and complexation (Q com) interactions. Based on the equilibrium experiments, montmorillonite, goethite, ferrihydrite and kaolinite were selected after adsorption of Pb2+ for stepwise extraction to quantify the adsorption mechanisms. In detail, 0.4 g of a sample was added into a centrifuge tube with 80 mL Pb2+ solution. When the adsorption process was complete, the adsorbent was washed with deionized water and the supernatant was removed. Following this, the prepared 20 mL of various extraction reagents were mixed with the Pb-loaded sample and were shaken for 24 h for Pb-loaded samples in deionized water and NaNO3 solution, for 2 h for samples in MgCl2 and for 5 h for samples in NaOAc. During the extraction process, the extracting reagents involved included deionized water, MgCl2 at pH 7, NaOAc at pH 5 and 0.01 M NaNO3. Finally, the contribution rate of each mechanism was calculated according to the adsorption/extraction capacity, and the effectiveness of the extracting reagent was obtained, which could be calculated according to Equations 1 & 2:
where C 0 and C e (mg L–1) correspond to the initial and equilibrium concentrations of Pb2+, Q (mg g–1) is the total amount of adsorption, Qi (mg g–1) is the adsorption capacity corresponding to each adsorption mechanism, R is the proportion of each adsorption mechanism, m (g) is the weight of dried adsorbent and V (L) is the Pb2+ solution content.
Adsorption kinetics model
Two models (pseudo-first order (Equation 3) and pseudo-second order (Equation 4)) were used to fit the kinetics experimental data:
where Q e and Qt (mg g–1) correspond to the adsorption capacities at equilibrium and time t (min), respectively, k1 and k2 represent the rate constants.
Adsorption isotherm model
The isotherm experimental data were fitted to two adsorption isotherm models (Freundlich (Equation 5) and Langmuir (Equation 6)), with the parameters presented in Table 1.
Table 1. Adsorption isotherm parameters of Pb2+ onto kaolinite, montmorillonite, goethite and ferrihydrite at pH 4–6.
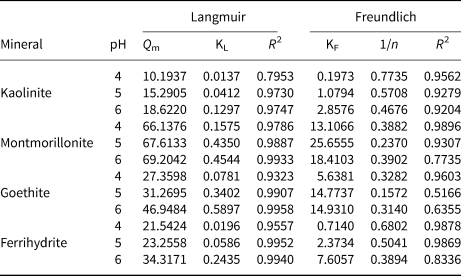
where C e (mg L–1) refers to the equilibrium concentration, Q e and Q m (mg g–1) are the equilibrium and saturated adsorption capacities, respectively, KL and KF are the adsorption rate constants implying the degree of adsorption, respectively, and 1/n is the heterogeneous factor.
Results and discussion
XRD characterization
The XRD traces of kaolinite, montmorillonite, goethite and ferrihydrite are shown in Fig. 1. These patterns matched well with the standard XRD traces of the four minerals. Information in the XRD traces allows identification of kaolinite, montmorillonite, goethite and ferrihydrite. No new reflections appeared after adsorption of Pb2+ by kaolinite, montmorillonite, goethite and ferrihydrite (Fig. 1), indicating that no new phase formed after the adsorption of Pb2+. However, Fig. 1b showed that the peak intensity of montmorillonite after adsorption was weakened and the layer spacing was decreased. Layer spacing is one of the important factors reflecting the structural characteristics of montmorillonite, which can be calculated using the Bragg equation (Yu et al., Reference Yu, Zeng, Wu, Wang and Liu2007). Specifically, the reflection that appeared at 5.86°2θ was consistent with the (001) plane of montmorillonite, and the corresponding layer spacing was 1.51 nm. The characteristic reflection located at 5.86°2θ was shifted to 6.17°2θ after the adsorption of Pb2+. The layer spacing on the d 001 surface of montmorillonite reduced from 1.51 to 1.43 nm after the adsorption of Pb2+, illustrating that Pb2+ adsorption led to variation in the montmorillonite layer spacing, which was also demonstrated in Qu et al. (Reference Qu, Du, Ma, Chen, Cai and Huang2018).
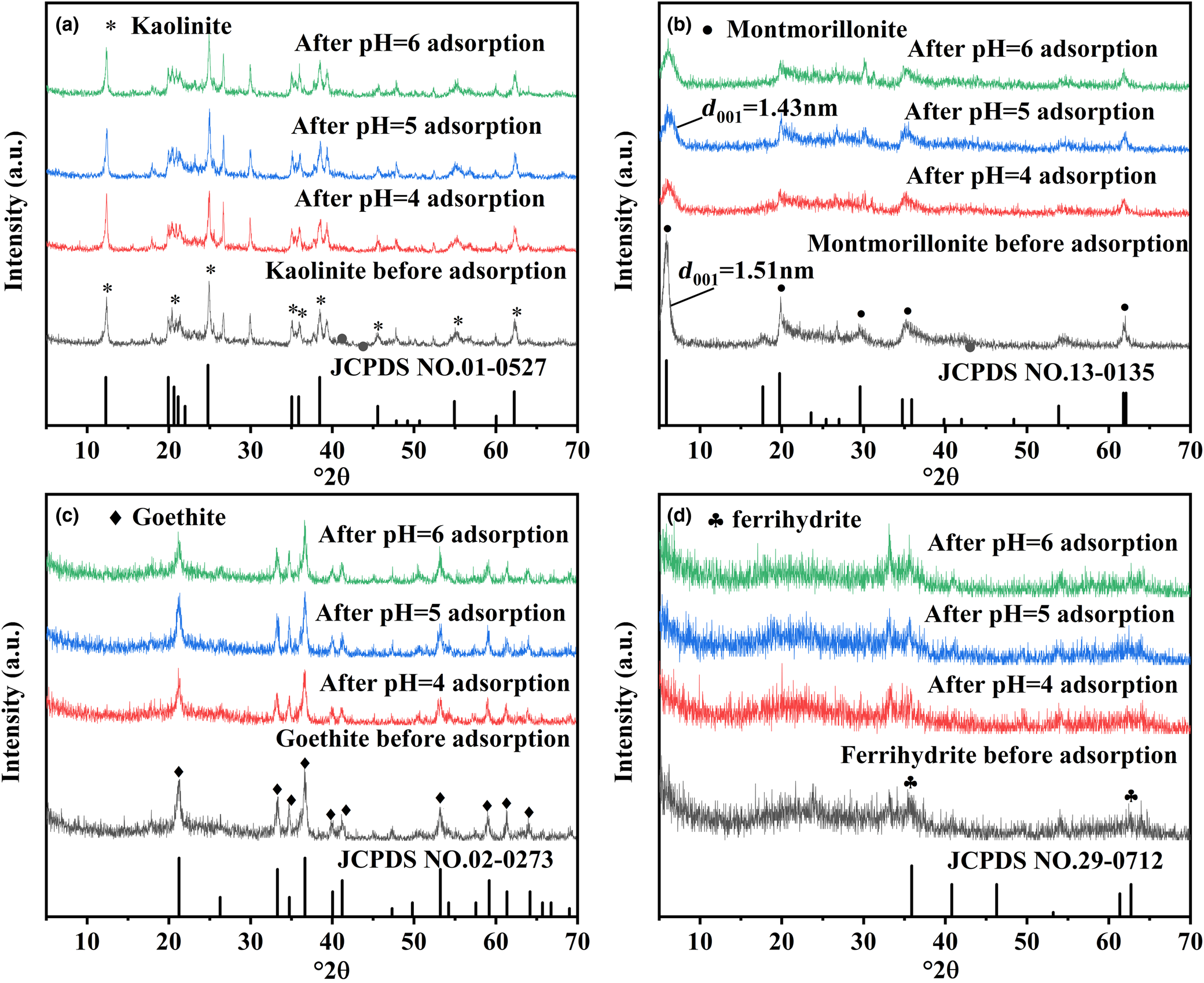
Fig. 1. XRD traces of (a) kaolinite, (b) montmorillonite, (c) goethite and (d) ferrihydrite before and after Pb2+ adsorption at pH 4–6.
SEM analysis
Kaolinite and montmorillonite both displayed a sheet-like morphology, while goethite and ferrihydrite showed a rod-like and irregular block-like morphology, respectively (Fig. 2a–h). The elemental composition of the four minerals was characterized using an energy-dispersive spectrometer (EDS) and elemental mapping. The results showed that the kaolinite presented a homogeneous distribution of Al, O and Si elements before adsorption (Fig. 2a,b). It was observed that O, Si, Al, Mg and Ca occurred in montmorillonite (Fig. 2c,d). Goethite and ferrihydrite were composed chiefly of Fe and O elements (Fig. 2e–h). No Pb element was found in the four minerals before adsorption, while the Pb element with various mass percentages of 5.16, 4.12, 2.84 and 3.47% was detected after adsorption. The above results confirmed that Pb2+ was adsorbed on the mineral surface.
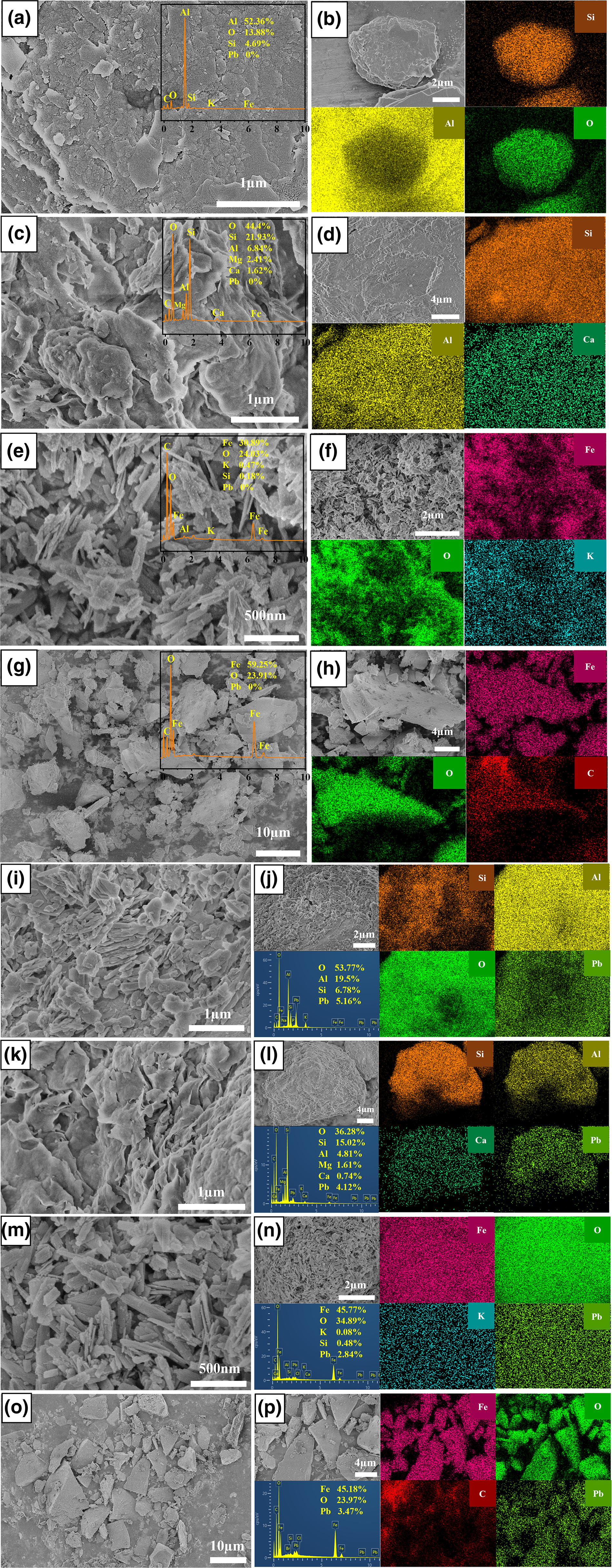
Fig. 2. SEM images, EDS spectra and elemental mapping images of the four minerals before and after Pb2+ adsorption. (a,b), (c,d), (e,f) and (g,h) represent kaolinite, montmorillonite, goethite and ferrihydrite before adsorption, respectively; (i,j), (k,l), (m,n) and (o,p) represent kaolinite, montmorillonite, goethite and ferrihydrite after adsorption at pH 6, respectively.
FTIR spectroscopy analysis
FTIR spectroscopy was used to investigate the interaction mechanisms among the four minerals (kaolinite, montmorillonite, goethite and ferrihydrite) and Pb2+ related to various functional groups. O–H, Si–O, Al–O and other functional groups exist in the four minerals, among which O–H plays an important role in the adsorption process. In the case of kaolinite, internal and surface stretching of –OH corresponded to the peaks between 3620 and 3695 cm–1 (Fig. 3a; Mouni et al., Reference Mouni, Belkhiri, Bollinger, Bouzaza, Assadi and Tirri2018). The intensities of all peaks caused by the –OH stretching modes were reduced due to the adsorption of Pb2+. For montmorillonite, the bands at 1635 and 3412 cm–1 demonstrated the existence of the bending and stretching vibrations of adsorbed water (Fig. 3b). Both bands were reduced significantly after Pb2+ adsorption. The adsorption band at 3620 cm–1 represented structural –OH stretching (Liu et al., Reference Liu, Yuan, Liu, Cai, Tan and He2013). The position of the structural –OH shifted to slightly greater wavenumbers after adsorbing Pb2+, indicating that the –OH group participated in the adsorption process of Pb2+ (Wu et al., Reference Wu, Zhang, Dai, Zhu, Dang and Li2011). Figure 3c shows that the adsorption peaks of goethite at 890 and 798 cm–1 were caused by the bending vibrations of Fe–OH, and the broad band at 3132 cm–1 illustrates the –OH stretching vibration of the group (Adebayo et al., Reference Adebayo, Adegoke and Fauzeeyat2020). The results demonstrate that the peaks near 3132, 890 and 798 cm–1 broadened, revealing that –OH groups participated in the adsorption process. Ferrihydrite presented bands at 3354 and 1044 cm–1 related to the stretching vibration of structural –OH and the bending vibration of Fe–OH, respectively (Fig. 3d; Yang et al., Reference Yang, Zhou, Li, Dou, Li and Wang2021). After the adsorption occurred, the peak located at 3354 cm–1 shifted to 3389 cm–1, while the band at 1044 cm–1 demonstrated reduced intensity. These indicate the interaction of Pb2+ with the hydroxyl group on ferrihydrite (Gan et al., Reference Gan, Shang, Li, Zhang and Fu2019).
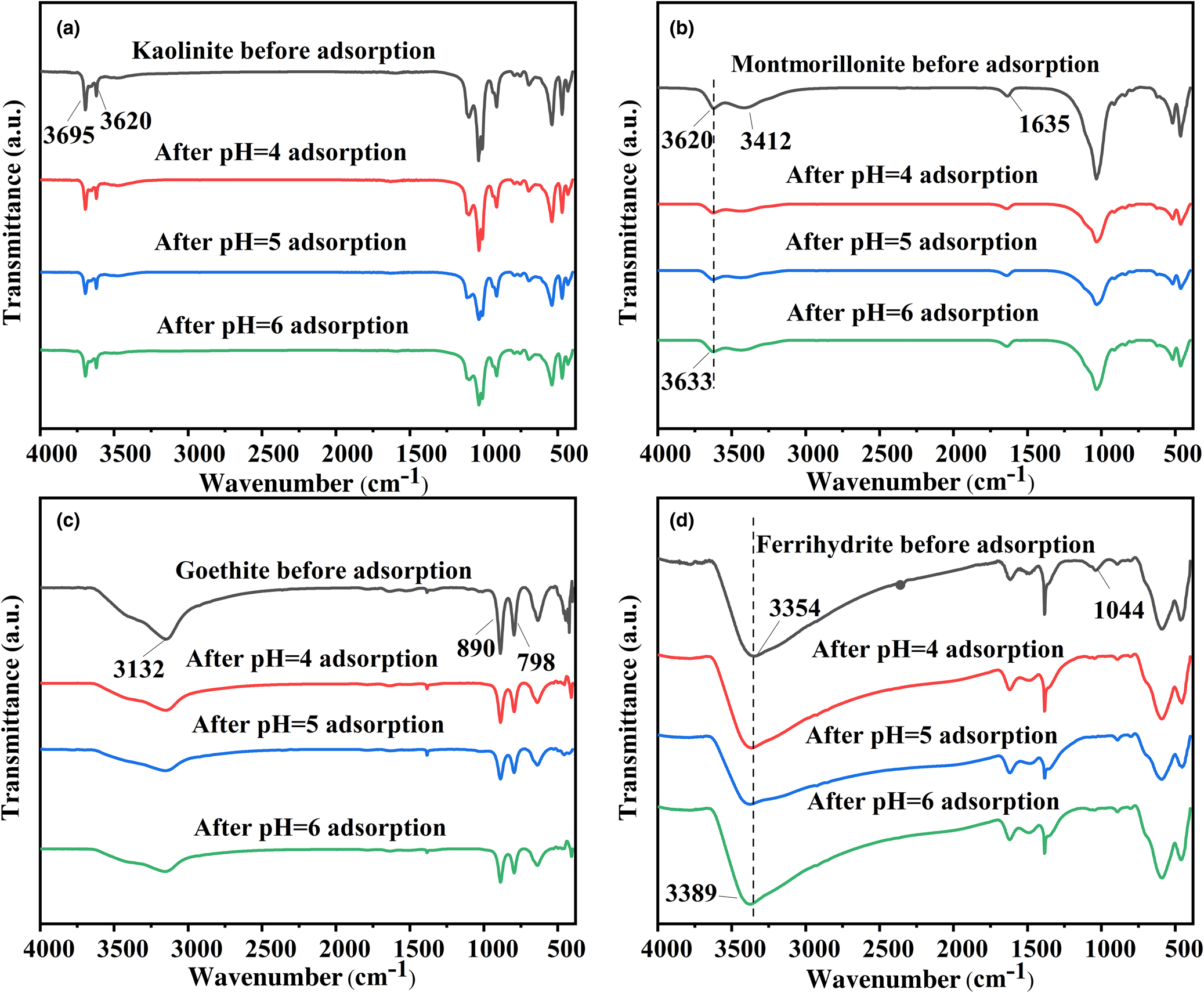
Fig. 3. FTIR spectra of (a) kaolinite, (b) montmorillonite, (c) goethite and (d) ferrihydrite before and after Pb2+ adsorption at pH 4, 5 and 6.
Adsorption kinetics
Figure 4 displays the relationship between contact time and the amounts of Pb2+ adsorbed by kaolinite, montmorillonite, goethite and ferrihydrite under batch experiments. The adsorption rates of the four minerals for Pb2+ were rapid, occurring within 5 min, and then gradually declined and became stable (Fig. 4). It has been speculated that the decreasing adsorptive sites occupied by Pb2+ reduce the adsorption rate (Zhang et al., Reference Zhang, Li, Li and Yang2017; Penido et al., Reference Penido, Melo, Guilherme and Bianchi2019). Notably, Pb2+ adsorption on montmorillonite and goethite reached equilibrium within 20 min, while Pb2+ adsorption on kaolinite and ferrihydrite took 60 min to reach equilibrium. The reason for this result might be the different mechanisms of adsorbing Pb2+ exhibited by the four minerals. The corresponding parameters are depicted in Table 2. The findings reveal that the adsorption process was more suitable for the pseudo-second order model (R 2 > 0.98), which indicates that Pb2+ adsorption on the four minerals was determined by chemisorption (Ahmad et al., Reference Ahmad, Gao, Mosa, Yu, Yin and Bashir2018; Jung et al., Reference Jung, Lee, Choi and Lee2019).
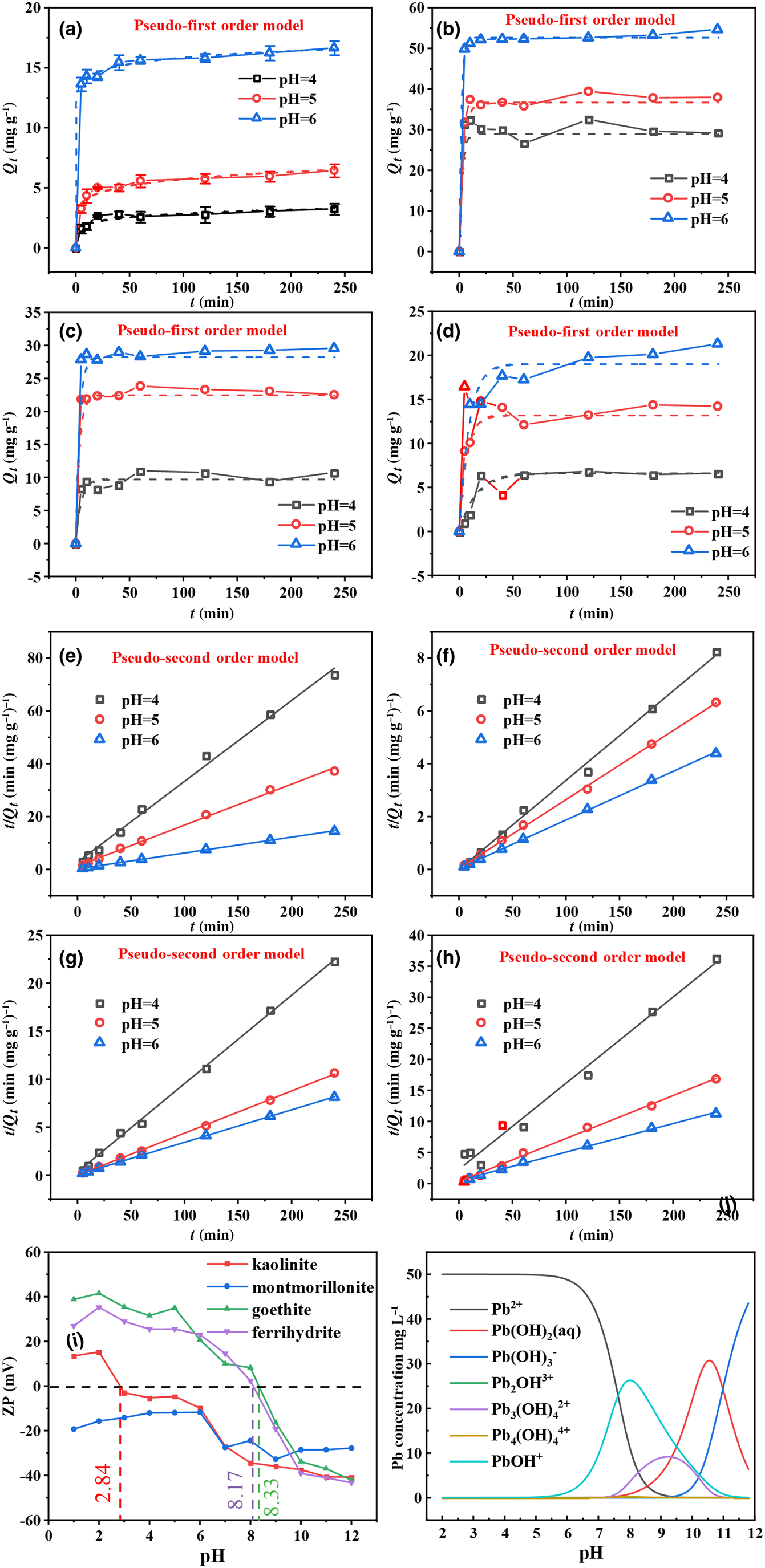
Fig. 4. (a–d) Pseudo-first order kinetics and (e–h) pseudo-second order kinetics of Pb2+ adsorption on kaolinite, montmorillonite, goethite and ferrihydrite at pH 4–6. (i) ζ-potentials (ZPs) of the four minerals under various pH conditions. (j) The species distributions of Pb2+ vs pH at the metal ionic concentration of 50 mg L–1.
Table 2. Adsorption kinetics parameters of Pb2+ onto kaolinite, montmorillonite, goethite and ferrihydrite at pH 4–6.
The pH value is a crucial factor in adsorption that can influence the surface charge of the adsorbent and the speciation of heavy metals in solution, which in turn affects the adsorption mechanism (Qu et al., Reference Qu, Tian, Jiang, Cao, Akindolie and Hu2020a). Therefore, the surface properties of the four minerals were further tested using a ζ-potential analyser. The points of zero charge (pHpzc) of kaolinite, goethite and ferrihydrite were determined to be ~2.84, 8.17 and 8.33, respectively, while montmorillonite was negatively charged at the tested pH values of 1–12 (Fig. 4i). It should be noted that lead presents primarily in the form of Pb2+ at the experimental pH values of 4–6 (Fig. 4j). Therefore, theoretically, montmorillonite and kaolinite demonstrated better Pb2+ adsorption performance than goethite and ferrihydrite, which might be due to electrostatic repulsion between goethite and ferrihydrite and the positive Pb2+ (Qu et al., Reference Qu, Tian, Jiang, Cao, Akindolie and Hu2020a). However, the amount of Pb2+ adsorption of the various minerals was in the following order: montmorillonite > goethite > ferrihydrite > kaolinite (Fig. 4a–d). The excellent adsorption properties of montmorillonite might be caused by its high electronegativity and typical cation-exchange capacity (Zhu et al., Reference Zhu, Chen, Zhou, Xi, Zhu and He2016). Nevertheless, the adsorption performance of kaolinite for Pb2+ was less impressive than that of goethite and ferrihydrite, although its pHpzc was lower. This result reveals that electrostatic interaction was not the dominant mechanism determining the adsorption capacities of goethite, ferrihydrite and kaolinite. In addition, the adsorption capacities of the four minerals for Pb2+ demonstrated an upward trend with increasing solution pH, which could be attributed to the precipitation and complexation of Pb2+ on the four minerals at greater pH values. This was consistent with the stepwise extraction results showing that pH was correlated positively with precipitation and complexation.
Adsorption isotherms
The adsorption isotherms of Pb2+ on the four minerals are shown in Fig. 5. The adsorption capacities of the four minerals increased rapidly with increasing Pb2+ concentration up to 50 mg L–1 and then increased more slowly to adsorption equilibrium. The adsorption properties of the four minerals for Pb2+ were compared. Note that montmorillonite showed the strongest adsorption performance for Pb2+ and kaolinite showed the weakest performance, which illustrates that Pb2+ adsorption on montmorillonite was outstanding. Moreover, the adsorption capacities of the four minerals were correlated positively with pH and increased with increasing pH, which was consistent with the kinetics results.
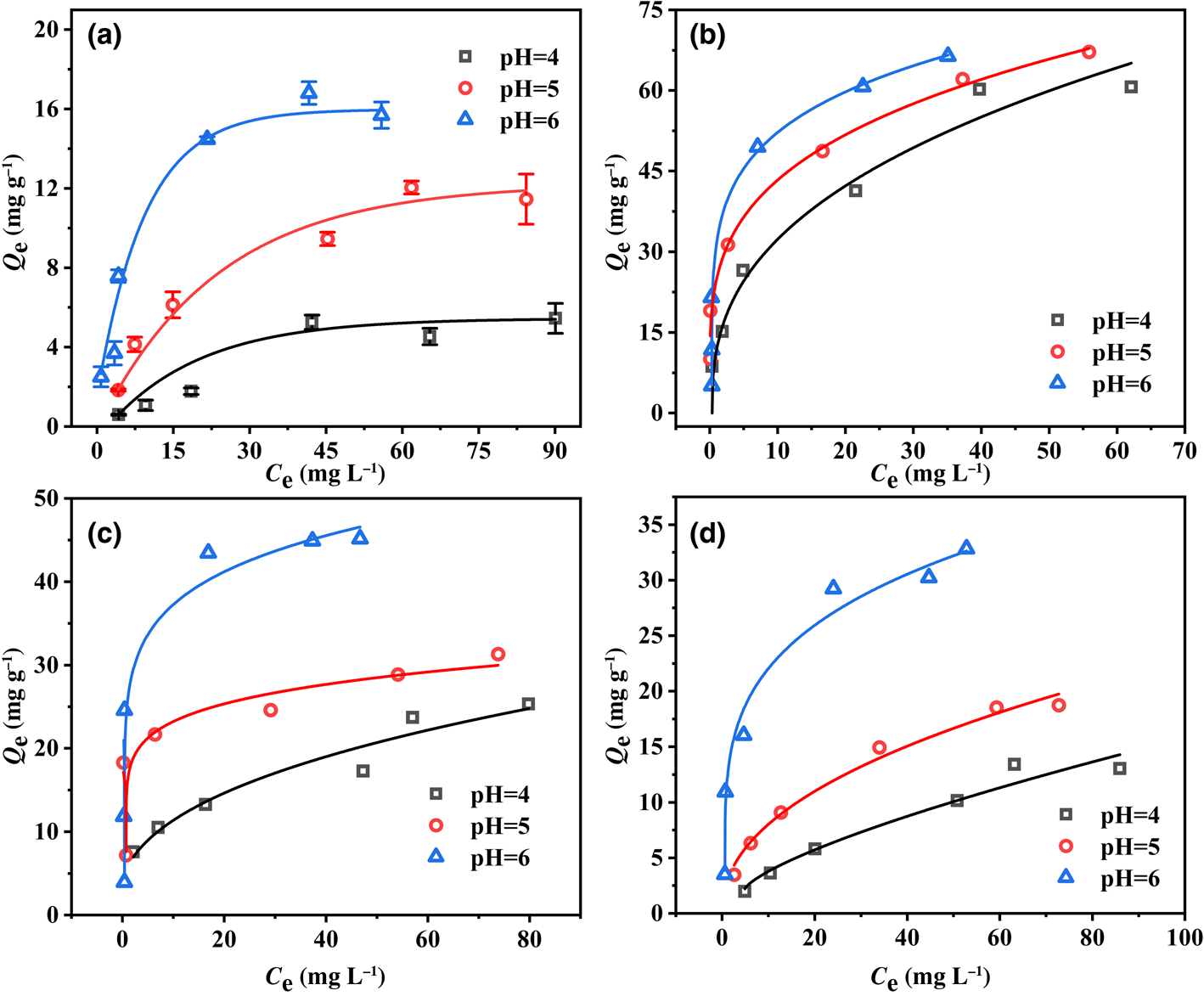
Fig. 5. Adsorption isotherms of Pb2+ on (a) kaolinite, (b) montmorillonite, (c) goethite and (d) ferrihydrite at pH 4–6.
Langmuir and Freundlich models were used to simulate the obtained data, and the corresponding calculated parameters are listed in Table 1. The results demonstrate that the Freundlich isotherm better fit the Pb2+ adsorption data as shown by the greater R 2 value at pH 4, which indicates that the adsorption processes of the four minerals were mainly heterogeneous and represent multilayer adsorption. The Langmuir model (0.97 < R 2 < 0.99) of the four minerals showed a better fit effect than the Freundlich model (0.51 < R 2 < 0.98) at pH 5 and 6, illustrating that Pb2+ adsorption on the four minerals might be ascribed to the monolayer coverage of Pb2+ and homogeneous sites on the surface of the four minerals (Al-Ghouti & Da'Ana, Reference Al-Ghouti and Da'Ana2020; Wu et al., Reference Wu, Wang, Wang, Zhang and Pan2021). In addition, the total adsorption capacity of montmorillonite for Pb2+ was greater than that of kaolinite, goethite and ferrihydrite at pH 4–6. The maximum adsorption ability achieved for Pb2+ was 69.20 mg g–1 with montmorillonite at pH 6, whereas adsorption abilities reached 18.62, 46.95 and 34.32 mg g–1 with kaolinite, goethite and ferrihydrite, respectively, which was attributed to the typical cation-exchange capacity of montmorillonite. A relatively weak pH dependence was observed for montmorillonite adsorption, indicating that the main mechanism of montmorillonite adsorption was not pH-dependent complexation but the cation exchange between Pb2+ and the interlayer cations of montmorillonite (Chen & Kocar, Reference Chen and Kocar2018).
Adsorption mechanism of Pb2+
The interactions that exist between soil minerals and metal ions are caused by multiple mechanisms (Gu et al., Reference Gu, Kang, Wang, Lichtfouse and Wang2019). To explore the Pb2+ adsorption mechanisms of the four minerals, the amount and contribution of each adsorption mechanism were quantified using a stepwise extraction method, and the results are depicted in Fig. 6. The contributions of physisorption for kaolinite, montmorillonite, goethite and ferrihydrite were 21.94–24.53, 1.02–1.87, 1.39–2.88 and 20.55–21.78% at pH 4–6, respectively. These results indicate that chemical action rather than physical action dominated the adsorption of Pb2+ by the four minerals. Regarding the adsorption process of Pb2+, the four minerals demonstrated various adsorption mechanisms. For montmorillonite, the contribution of ion-exchange mechanism (R exc) values ranged from 49.24 to 66.73% and decreased with increasing pH, indicating that ion exchange was the principal mechanism. Kaolinite, goethite and ferrihydrite all had smaller R exc values than 18.91% and their contribution of complexation (R com) values were greater than 38.38%, which imply that the adsorption mechanism of kaolinite, goethite and ferrihydrite was complexation rather than ion exchange. The adsorption mechanism determines the occurrence state of Pb2+ in soil, which affects the stability of the adsorbed Pb2+ accordingly. The Pb2+ adsorbed on the four minerals via physical interaction and cation exchange is unstable and highly bioavailable. By contrast, Pb2+ adsorbed through precipitation represents the partially bioavailable fraction, and the Pb2+ adsorbed through complexation is stable and harmless in soils. In brief, the great capacity of montmorillonite to adsorb Pb2+ was primarily due to the action of ion exchange, which only changes the occurrence state of Pb2+ and does not reduce environmental risk. The removal of Pb2+ via complexation with montmorillonite, kaolinite, goethite and ferrihydrite could reduce the readily bioavailable Pb2+, thereby decreasing environmental risks.
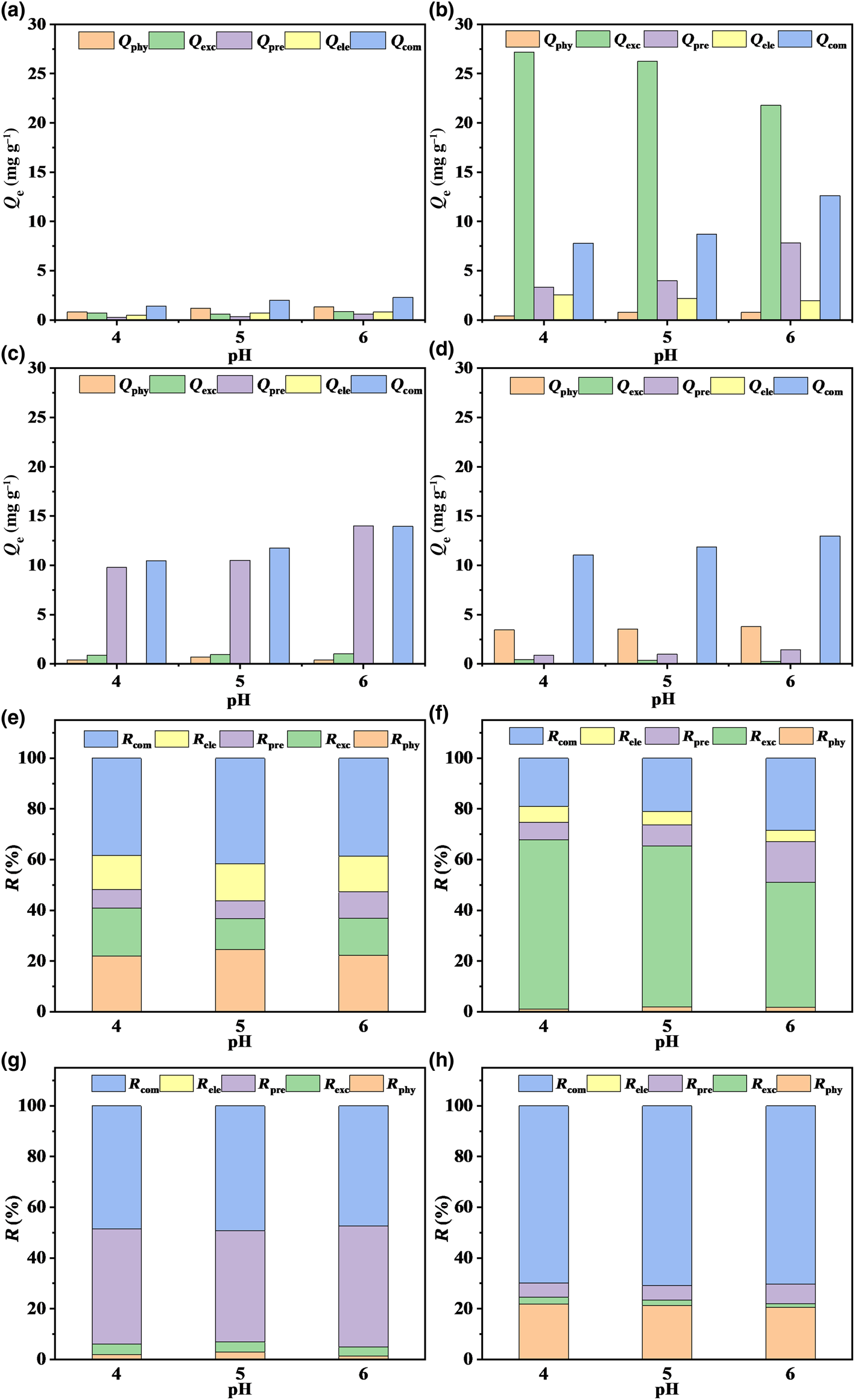
Fig. 6. Quantitative analysis of the (a–d) adsorption capacities and (e–h) contribution rates of the adsorption mechanisms of Pb2+ onto kaolinite, montmorillonite, goethite and ferrihydrite. Adsorption capacities are Q phy for physisorption, Q exc for ion exchange, Q pre for precipitation, Q ele for electrostatic and Q com for complexation; contribution rates are R phy for physisorption, R exc for ion exchange, R pre for precipitation, R ele for electrostatic and R com for complexation.
The influence of pH on the Pb2+ adsorption of the four minerals was explored at an initial pH of 4–6. Figure 6a–d demonstrates that the adsorption capacity of montmorillonite was ~13.0, 1.5 and 2.5 times those of kaolinite, goethite and ferrihydrite, respectively, at pH 4–6, indicating the excellent adsorption performance of montmorillonite. The pH value of the solution affected considerably the contributions of various adsorption mechanisms, especially for montmorillonite (Pehlivan et al., Reference Pehlivan, Özkan, Dinç and Parlayici2009). The pH value was correlated negatively with ion exchange and correlated positively with precipitation and complexation, which was attributed primarily to the increased pH and easier formation of metal hydroxides and carbonates (Fig. 6b; Zhong et al., Reference Zhong, Chen, Li, Ding, Liu and Liu2020). The contributions of electrostatic interactions to Pb2+ absorption on kaolinite and montmorillonite at pH 6 were 14.16 and 4.41%, respectively, while those for goethite and ferrihydrite were 0%. This was because kaolinite and montmorillonite were negatively charged at the experimental pH, while goethite and ferrihydrite were positively charged. Therefore, electrostatic interaction was involved in the uptake of Pb2+ by kaolinite and montmorillonite. Thus, pH value not only affected the adsorption mechanisms of the four minerals for Pb2+, but also had an effect on the stability of the adsorbed Pb2+. In general, considering the contributions of Pb2+ adsorption on the four minerals, it could be determined that pH value had the most critical effect on the Pb2+ adsorption process.
Conclusion
The Pb2+ adsorption capacities of kaolinite, montmorillonite, goethite and ferrihydrite were investigated. Furthermore, the contributions of various adsorption mechanisms at various pH values to remove Pb2+ were quantified using stepwise extraction experiments. The results illustrated that the four minerals had a significant capacity for Pb2+ removal. The kinetics of Pb2+ adsorption by the four minerals could be described well by the pseudo-second order model, indicating that the Pb2+ adsorption by the four minerals occurred mainly via chemical adsorption. Solution pH values affected the contributions of the various adsorption mechanisms considerably, especially for montmorillonite. Regardless of solution pH, the adsorption mechanisms of Pb2+ by montmorillonite and ferrihydrite were primarily ion exchange and complexation, accounting for 49.24–66.73 and 69.87–70.90%, respectively. The principal Pb2+ absorption mechanisms of kaolinite were physical adsorption and complexation with a combined contribution of 60.32–66.23%, while those of goethite were precipitation and complexation with a combined contribution of 93.17–95.08%. These results, especially those from the stepwise experiments, improve our understanding of the fate of Pb2+ in soils.
Financial support
This research was supported by the Fundamental Research Funds for the Central Universities (PA2019GDQT0009).
Conflicts of interest
The authors declare none.












