Accepted for publication Mar 15, 2023.
This article has been co-published in The Annals of Thoracic Surgery and Cardiology in the Young.
The Society of Thoracic Surgeons requests that this article be cited as: Tretter JT, Spicer DE, Franklin RCG, et al. Expert Consensus Statement: Anatomy, Imaging, and Nomenclature of Congenital Aortic Root Malformations. Ann Thorac Surg. 2023; in press.
The Supplemental Tables, Figures, and Videos can be viewed in the online version of this article [https://doi.org/10.1016/j.athoracsur.2023.03.023] on https://www.annalsthoracicsurgery.org.
Over the last 15 years, several schemes have been proposed for categorization of abnormalities of the aortic root, particularly the so-called bicuspid aortic valve and its associated aortopathies.Reference Sievers and Schmidtke1–Reference Tretter, Izawa and Spicer4 Most have been written by specialists treating adult patients with otherwise normal hearts. These fall short when seeking to correlate the relevant 3-dimensional complexity of the aortic root with accurate and logical classification schemes capable of accounting for the entire spectrum encountered when the root is congenitally malformed.Reference Sievers and Schmidtke1–Reference Michelena, Della Corte and Evangelista3 We offer here a perspective from the stance of specialists in congenital cardiac disease. We based our approach on an understanding of normal and abnormal morphogenesis and anatomy, with emphasis placed on the features of clinical and surgical relevance. We anticipate that our suggested terms and definitions will be of value across all cardiac specialties, whether pediatric or adult, and will prove of equal value in describing acquired and congenital cardiac disease.
Anatomy of the normal aortic root
The aortic root is a complex, 3-dimensional, structure extending proximally from the virtual basal ring to the sinutubular junction distally, made up of 3 individual leaflets, each attached within their supporting sinus in semilunar fashion, with the sinuses separated by the interleaflet triangles (Supplemental Table 1).Reference Anderson5 Each leaflet is attached distally at the sinutubular junction, and its nadir is at the level of the virtual basal ring (Figure 1). The overall arrangement produces a crown-like configuration.
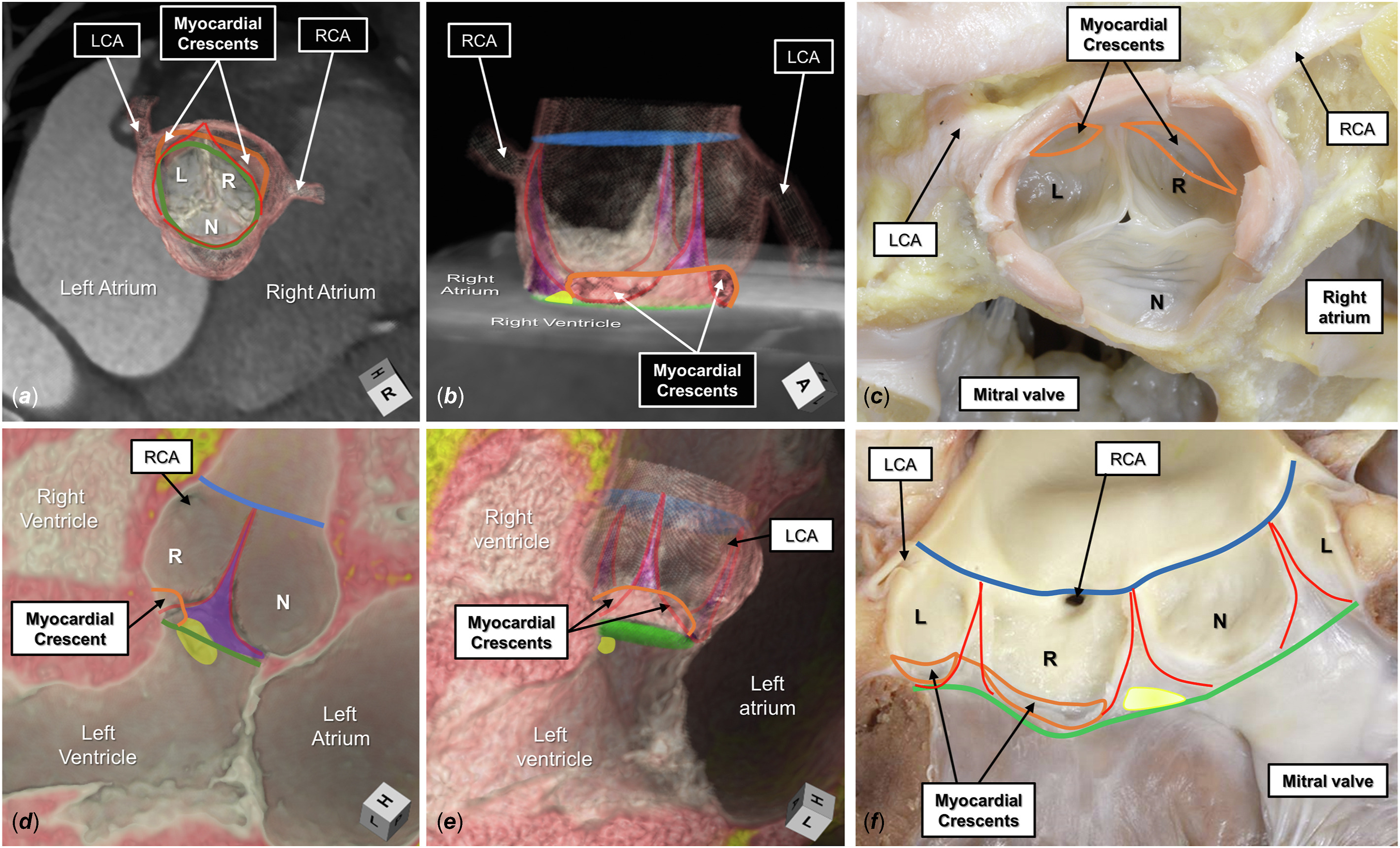
Figure 1. The normal aortic root is shown by 3-dimensional computed tomographic reconstruction in its (A) short axis and (B) long axis, viewing the myocardial-arterial junction incorporated between the coronary sinuses (orange line). The semilunar lines of attachment, in red, extend from the sinutubular junction, colored blue, to the level of the virtual basal ring, colored green. The interleaflet triangles are colored purple. In panel B, the superior aspect of the membranous septum is colored yellow and merges with the base of the interleaflet triangle separating the right (R) and noncoronary (N) aortic sinuses. (C) A comparable autopsied heart specimen. (D) A virtual dissection of a long-axis plane of the root. (E) The entirety of the root is added. (F) The comparable autopsied heart. (L, left coronary aortic sinus; LCA, left coronary artery; RCA, right coronary artery.)
During diastole, 3 peaks of the crown, usually named as commissures, can be identified at the attachments of the leaflets at the sinutubular junction. The zones of apposition between the leaflets then extend radially from the commissures to the centroid of the valvar orifice.Reference Tretter, Spicer, Mori, Chikkabyrappa, Redington and Anderson6 The space proximal to the skirt of tissue provided by the leaflets during diastole, which is distal to the plane of the virtual basal ring, hemodynamically is part of the left ventricular outflow tract.Reference Tretter, Spicer, Mori, Chikkabyrappa, Redington and Anderson6,Reference Suzuki, Mori and Izawa7
The surface areas of coaptation span between the centrally positioned nodules of Arantius and the peripherally positioned commissures. The areas of apposition on either side of these nodules are known as the lunules, with the greatest surface area of coaptation found midway within each lunule.Reference Tretter, Izawa and Spicer4,Reference De Kerchove, Momeni and Aphram8 Noncoapting areas, the bellies, extend to the margins of the semilunar hinges and serve as the interface of the hemodynamic ventriculoarterial junction in diastole. The commissures form the apexes of the interleaflet triangles, with the triangles themselves forming the walls of the root belonging to the left ventricular outflow tract (Figure 2).

Figure 2. (A, C) Three-dimensional computed tomographic reconstructions of a normal aortic root during diastole. The 3 zones of apposition are colored white with black borders, comprising the components of the leaflets marked in panel E. The midpoint of coaptation is positioned just over halfway between the sinutubular junction, colored blue, and midportion of the root (white double-headed arrow), closer to the virtual basal ring, colored green. The zone of apposition increases in its midportion (blue double-headed arrows), before decreasing towards the commissures (white stars with red borders). The hemodynamic ventriculoarterial junction, formed by the leaflet bellies, is colored red, bordered peripherally by their semilunar attachments (red lines). This junction has 3 points peripherally at the commissures and a fourth shorter, central peak. The height of this central peak represents the difference between the effective height of the leaflets vs the coaptation length, the latter marked with a black double-headed arrow. (B, D) Comparable anatomy in an autopsied heart specimen. The lunules of adjacent coapting leaflets are labeled with corresponding numbers. (E) Components of the leaflets. (LCA, left coronary artery; RCA, right coronary artery.)
The virtual basal ring, or echocardiographic “annulus,” is the geometric planar surface, limited by the confines of the aortic root, created by joining the nadirs of attachment of the leaflets. It marks the proximal anatomical boundary of the aortic root. The sinutubular junction forms the distal boundary between the root and the ascending aorta (Figures 1, 2).Reference Tretter, Spicer, Mori, Chikkabyrappa, Redington and Anderson6
The virtual basal ring should not be equated with the ventriculoarterial junction. This latter junction, better described as being myocardial-arterial, is usually found only in the sinuses giving rise to the coronary arteries and their intervening interleaflet triangle. It is crossed by the semilunar line of the attachment of the leaflets, with marked individual variation. The walls of the noncoronary aortic sinus, in contrast, lack myocardial support. This sinus is supported by the central fibrous body, and the fibrous curtain with the aortic leaflets of the mitral valve (Figure 1).Reference Tretter, Spicer, Jacobs and Anderson9,Reference Toh, Mori and Tretter10 This fibrous curtain, spanning between the left and right fibrous trigones, also supports a variable portion of the left coronary leaflet.Reference Tretter, Mori and Saremi11,Reference Amofa, Mori and Toh12 The arrangement in the aortic root contrasts with the situation found in the pulmonary root, in which ventricular myocardium is incorporated into the bases of all 3 of its sinuses.Reference Tretter, Izawa and Spicer4,Reference Tretter, Spicer, Mori, Chikkabyrappa, Redington and Anderson6
When discussing the components of the roots, we have purposely avoided using the word “cusp.” This word is currently used to account for either the leaflets or the sinuses, or a combination of both.Reference Sievers, Hemmer and Beyersdorf13,Reference Anderson, Spicer, Quintessenza, Najm and Tretter14 If used, we suggest this should be confined to a description of the leaflets. The interleaflet triangles are the fibrous walls of the root beneath the 3 peaks of the valvar crown.Reference Sutton, Ho and Anderson15 The triangle between the right and noncoronary aortic sinuses is in continuity proximally with the membranous septum,Reference Tretter, Spicer, Mori, Chikkabyrappa, Redington and Anderson6,Reference Sutton, Ho and Anderson15–Reference Mori, Tretter and Toba18 which is usually positioned such that part is distal to the virtual basal ring.Reference Amofa, Mori and Toh12,Reference Tawara19 In most individuals, it is divided on the right side into atrioventricular and interventricular components by the attachment of the septal leaflet of the tricuspid valve.Reference Tretter, Mori and Saremi11 In the setting of the normal trisinuate aortic root, it is almost always accurate to describe left and right coronary aortic sinuses. Should the coronary arterial origin be anomalous, these sinuses are better described as facing, or adjacent to, the pulmonary root. This permits a logical description should a coronary artery arise from the nonadjacent sinus.
The central fibrous body is an integral part of the ventricular support of the root.Reference Tawara19 Usually described as comprising the membranous septum and the right fibrous trigone,Reference Zimmerman and Bailey20 it possesses a third part, namely, the roof of the inferoseptal recess of the left ventricular outflow tract. This area of fibrous continuity between the leaflets of the mitral and tricuspid valves supports the base of the buttress of the atrial septum. Its zone of continuity with the membranous septum is penetrated by the atrioventricular conduction axis.Reference Macías, Tretter and Sánchez-Quintana21 The nonbranching bundle, positioned on the crest of the muscular septum, then courses along the inferior margin of the membranous septum, where it gives rise to the right and left bundle branches. The superior fascicle of the left bundle often ascends the crest of the muscular ventricular septum toward the nadir of the right coronary leaflet before slowly descending and wrapping around the left ventricular outflow tract toward the base of the superolateral papillary muscle.Reference Tawara19,Reference Macías, Tretter and Sánchez-Quintana21 It is appreciation of both the variability seen in the relationship of the inferior margin of the membranous septum relative to the plane of the virtual basal ringReference Amofa, Mori and Toh12 and the common adjacency of the left bundle branch to the nadir of the right coronary leaflet that can guide the surgeon to avoid damage to the ventricular components of the conduction axis.Reference Tretter, Izawa and Spicer4,Reference Tawara19,Reference Macías, Tretter and Sánchez-Quintana21
Anatomy of the congenitally malformed aortic root
Description is greatly simplified when the components are assessed in terms of the number of sinuses present. Most frequently, the malformed root retains the trisinuate arrangement, although in a minority of cases it is bisinuate, or even more rarely, quadrisinuate (Figure 3; Supplemental Table 2).
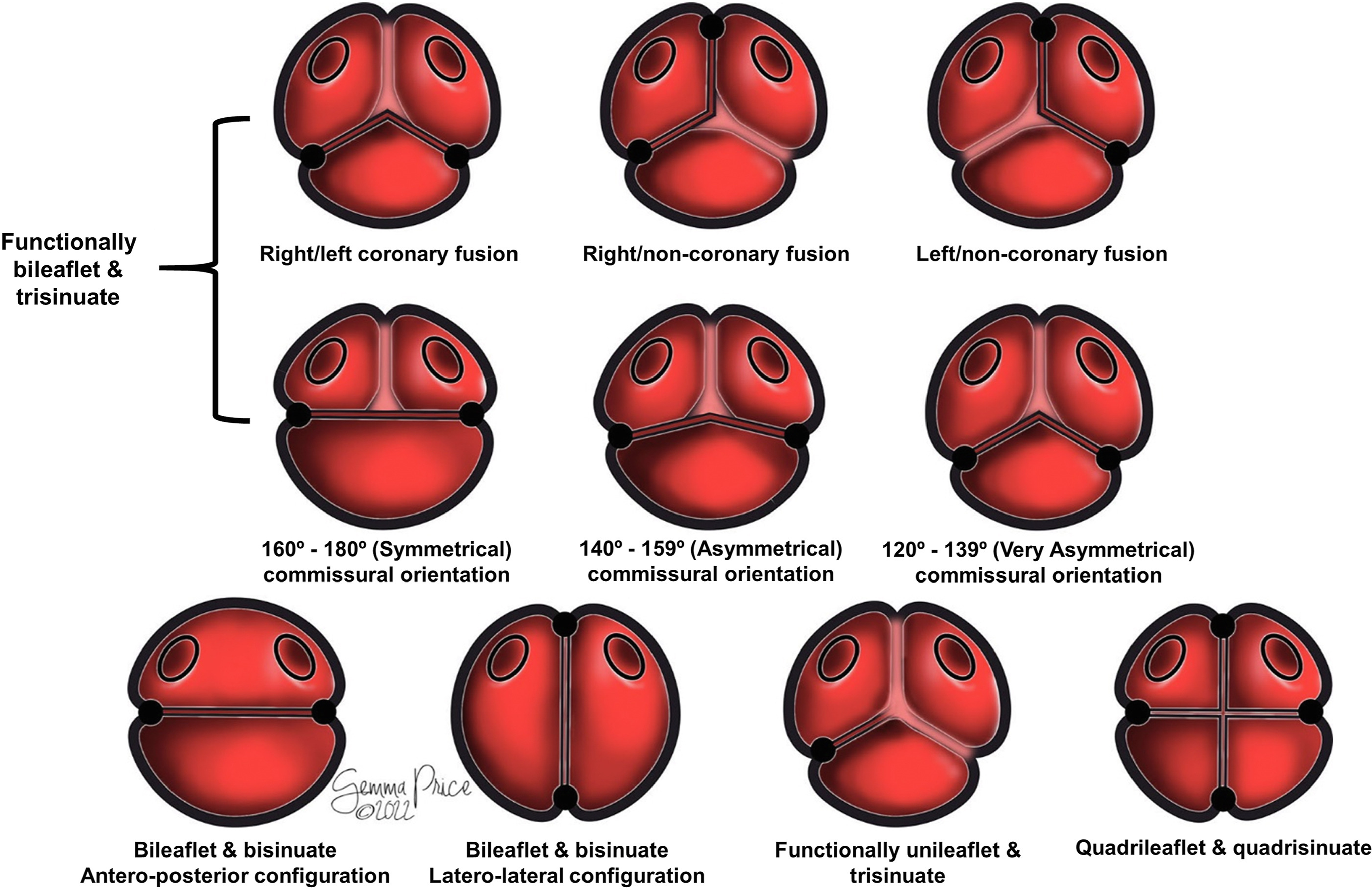
Figure 3. The drawing shows how classification of the congenitally malformed aortic root is simplified when described in terms of leaflets, sinuses, and interleaflet triangles.
Functionally bileaflet aortic valve
The functionally bileaflet, or “bicuspid,” aortic valve is found in almost nine-tenths of all congenitally malformed roots.Reference Sabet, Edwards, Tazelaar and Daly22,Reference Sillesen, Vøgg and Pihl23 Possessing 3 leaflets, sinuses, and interleaflet triangles, its presence reflects the fusion, during development, of 2 of the leaflets, with a resulting raphe. The corresponding interleaflet triangle is hypoplastic, no longer extending distally to reach the sinutubular junction. The “raphe” is the prominent tissue found at the site of fusion between the leaflets and can be variable in its extent. Fused variants have been described in its absence.Reference Michelena, Della Corte and Evangelista3 This could reflect ambiguity in the use of “raphe,” or indicate that the lesions are bisinuate rather than trisinuate. We have yet, in our collective experiences, to encounter a trisinuate root with 2 functional leaflets lacking a raphe. When a zone of fusion is present, our evidence suggests that a raphe will be present, even if minimally formed. Should ambiguity persist, attention should be turned toward long-axis echocardiographic imaging and cross-sectional interrogation of the hypoplastic interleaflet triangle, which would be present in the trisinuate but not the bisinuate variant (Supplemental Figures 1, 2).
Incomplete fusion of less than half of the zone of apposition is commonly considered a “partial,” or “forme fruste” bicuspid valve. We have found an inverse relationship between the length of the zone of fusion and the corresponding hypoplasia of the interleaflet triangle, with a spectrum from zero to complete (Figures 4A, 4B). This feature, along with asymmetry between the conjoined and third leaflets, dictates the plane of opening of the valvar orifice area relative to the long axis of the ascending aorta and to the plane of the sinutubular junction. Such asymmetry is pronounced compared with the normal aortic root.Reference Tretter, Spicer, Mori, Chikkabyrappa, Redington and Anderson6,Reference Sabet, Edwards, Tazelaar and Daly22 The degree of asymmetry is held to guide the type of surgical repair, as well as predicting its durability.

Figure 4. (A, B) Two different functionally bileaflet aortic valves. (A) Partial fusion between the right and noncoronary leaflets, with the zone of fusion (black double-headed arrow) representing <50% of the zone of apposition. (B) A functionally bileaflet aortic valve with greater fusion between the coronary leaflets and lower height of the corresponding interleaflet triangle (yellow caret). This reduces the aortic valvar opening area and tilts its plane at a greater angle relative to that of the sinutubular junction. (C) The bileaflet aortic valve with bisinuate aortic root. There are 2 leaflets, sinuses, and normal size interleaflet triangles, both with their apex reaching to the plane of the sinutubular junction (red dotted line). (D) A quadrileaflet and quadrisinuate aortic root, also without fusion between any of the leaflets, and therefore functioning as a quadrileaflet valve.
In the so-called symmetrical arrangement, with the 2 commissures positioned directly opposite each other at 160° to 180°, the surgical recommendation is usually an approach which maintains its “bicuspid” form. When the commissures are at 120° to 139° from each other, the arrangement is said to be “very asymmetric,” lending itself to “tricuspidization.” The intermediate position is deemed “asymmetric” (Figure 3, middle row). Depending on the strategy used for repair, and the commissural orientation, repositioning of the commissures has been demonstrated to improve valvar function and durability.Reference de Kerchove, Mastrobuoni and Froede24
It is most usually the right and left coronary leaflets that are fused, followed by the right and noncoronary leaflets, and rarely, the left and noncoronary leaflets (Supplemental Figures 1A, 1B).Reference Sievers and Schmidtke1,Reference Sabet, Edwards, Tazelaar and Daly22 The rarity of the third variant may relate to the varied rotational position of the aortic root, and the corresponding variability in the underlying myocardial vs fibrous support.Reference Tretter, Mori and Saremi11,Reference Amofa, Mori and Toh12
When preparing for surgical repair, note should be taken of the height of the interleaflet triangles, which can accurately be measured using 3-dimensional imaging with multiplanar reformatting.Reference Tretter, Izawa and Spicer4,Reference Hagendorff, Evangelista, Fehske and Schäfers25 The leaflets themselves can be thickened, calcified, or fenestrated, as well as being compromised by subcommissural fusion. Should any components of the root be hypoplastic, this should be described (Supplemental Table 3).Reference Tretter, Izawa and Spicer4
Bileaflet and bisinuate aortic valve
A minority of malformed roots, less than one-tenth, are built on a bisinuate scaffold.Reference Sillesen, Vøgg and Pihl23 These phenotypes are more frequent in syndromes, particularly in Turner syndrome, which has the highest penetrance of bicuspid aortic valves, regardless of the valvar phenotype.Reference Klásková, Zapletalová and Kaprálová26 The root with 2 leaflets and 2 sinuses has only 2 interleaflet triangles, each of normal height, and hence equating to 2 normal commissures (Figure 4C). The normal height of the interleaflet triangles provides an orifice relatively parallel to the normal plane of the sinutubular junction.Reference Tretter, Spicer, Mori, Chikkabyrappa, Redington and Anderson6 This feature probably accounts for the lower propensity for ascending aortic dilation when compared with the functionally bileaflet variant. The paired leaflets and sinuses can be positioned anteroposteriorly or laterolaterally (Supplemental Figures 1C, 1D). The leaflets can be additionally malformed, be associated with subvalvar lesions impacting on leaflet motion, or the root itself can be hypoplastic.Reference Tretter, Izawa and Spicer4
Functionally unileaflet aortic valve
The functionally unileaflet, or “unicuspid,” variant is built on a trisinuate scaffold. Considered rare in the adult populations,Reference Novaro, Mishra and Griffin27,Reference Roberts and Ko28 its true prevalence is likely underestimated due to misdiagnosis as a functionally bileaflet valve.Reference Roberts and Ko28,Reference Slostad, Witt and O’Leary29 In newborns, it is the commonest form of critical aortic stenosis.Reference Tretter, Spicer, Mori, Chikkabyrappa, Redington and Anderson6 Its leaflets show 2 zones of fusion, each with a corresponding raphe and hypoplasia of the corresponding interleaflet triangle. Most commonly, the solitary zone of apposition is between the noncoronary and left coronary leaflets, with the solitary commissure positioned above the aortic-mitral curtain (Figure 5; Supplemental Video 1).Reference Tretter, Spicer, Mori, Chikkabyrappa, Redington and Anderson6 The lines of attachment of the conjoined leaflets are much closer to being truly annular.Reference Tretter, Steffensen, Westover, Anderson and Spicer30 When found in neonates or infants, the leaflets tend to be severely thickened (Figures 5A, 5B), but in adolescents or adults, there tends to be less complete fusion between the leaflets, with a greater opening area, and a less annular line of leaflet attachment (Figures 5C, 5D; Supplemental Figure 3).Reference Tretter, Spicer, Mori, Chikkabyrappa, Redington and Anderson6 Either way, the 3-dimensional plane of the aortic valvar opening area is at a significantly increased angle relative to the long axis of the ascending aorta compared with the functionally bileaflet form (Supplemental Figure 3C; Supplemental Video 2).Reference Tretter, Izawa and Spicer4 This results in an increased perturbance of flow.Reference Entezari, Schnell and Mahadevia31

Figure 5. Two functionally unileaflet and trisinuate aortic roots are shown with fusion between the right and left, and right and noncoronary leaflets (black dots mark the zones of fusion, and the yellow dot with the black border marks the normal commissure). (A, B) A greater degree of fusion is seen in this heart between both pairs of leaflets, with smaller interleaflet triangles. (C, D) The corresponding interleaflet triangles in this heart are larger. (A, B) Much thicker aortic valvar leaflets are additionally demonstrated. This combination results in a much smaller aortic valvar opening area. The difference in interleaflet triangle heights dictates the degree of inferior tilting of the aortic valvar opening area of the involved adjacent leaflets. (A, D) The red arrows mark the left coronary artery. The yellow dotted line represents the sinutubular junction.
In this light, it becomes intuitive that studies support early progression toward valvar dysfunction and dilation of the thoracic aorta in those with a functionally unileaflet aortic valve.Reference Mookadam, Thota and Garcia-Lopez32 These theories, however, require further investigation. Misdiagnosis may relate to the through-plane motion of 2-dimensional echocardiography, which can suggest that the zone of apposition has been interrogated in its entirety.Reference Ewen, Karliova and Weber33 To avoid this problem, it is advantageous also to use 3-dimensional echocardiography (Supplemental Video 3).Reference Hagendorff, Evangelista, Fehske and Schäfers25 If being considered for surgical repair, all features should all be interrogated by 3-dimensional imaging with multiplanar reformatting.Reference Tretter, Izawa and Spicer4,Reference Hagendorff, Evangelista, Fehske and Schäfers25 The leaflets themselves are usually abnormal, particularly when encountered in neonates, and are usually associated with hypoplasia of the aortic root.Reference Tretter, Izawa and Spicer4
Quadrileaflet aortic valve and its functional counterparts
The quadrileaflet, or “quadricuspid,” variant is extremely rare,Reference Tretter, Mori, Spicer and Anderson34 accounting for 0.005% of adults undergoing cardiac imaging.Reference Manuel, Ladeiras-Lopes and Ribeiro35 The quadrisinuate root, without fusion between the leaflets, functions as the name suggests (Figure 4D). Fusion, nonetheless, can produce functionally trileaflet, bileaflet, and unileaflet variants. The functionally trileaflet valve within a quadrisinuate root, however, is markedly different from the normal trileaflet and trisinuate root. There can be marked asymmetry between the leaflets and sinuses. The coronary arteries most often arise from adjacent aortic sinuses, although they can arise from opposite aortic sinuses.Reference Tretter, Mori, Spicer and Anderson34 The wide variation does not lend itself to alpha-numeric classification. Description is the better approach. The variability in location of the coronary arteries defeats any description of “coronary aortic sinuses.”Reference Tretter, Mori, Spicer and Anderson34 Three-dimensional imaging provides the information needed for surgical repair.Reference Tretter, Izawa and Spicer4,Reference Hagendorff, Evangelista, Fehske and Schäfers25 As with the other phenotypes, description should additionally include the leaflet substrate, any subvalvar substrate impacting leaflet motion, and whether the aortic root is hypoplastic.Reference Tretter, Izawa and Spicer4 This descriptive approach can also be applied to the variable truncal valvar morphologies seen in those born with a common arterial trunk.
Additional congenital and acquired abnormalities
Attention should be directed in all variants toward the presence of thickening or nodularity, perforation,Reference Hill, Bansal, Razzouk, Liu, Bailey and Gundry36 fenestration,Reference Reade, Szeto and Bavaria37 elongation, retraction, bending,Reference Soga, Takaya, Mori, Nishii and Hirata38 prolapse, flail, or billowing of the leaflets. A leaflet may rarely be absentReference Tretter, Steffensen, Westover, Anderson and Spicer30 or duplicated (Supplemental Table 3). If present, the location of the associated calcification should be described. Calcification along a zone of apposition can produce acquired fusion, thus mimicking congenital fusion.Reference Pawade, Sheth, Guzzetti, Dweck and Clavel39,Reference Jilaihawi, Makkar and Kashif40 Dilation can involve any part of the root or the ascending aorta. Abnormal function can reflect obstruction to flow, with stenosis within the left ventricular outflow tract or at the level of the leaflets or sinutubular junction, or incompetency of the valve.
Mention is required of the aortoventricular tunnel. This channel bypasses the attachment line of a leaflet, producing a passage from the valvar sinus into the left or right ventricle. This can be accompanied by bileaflet, dysplastic, or ectopic aortic valvar tissue.Reference McKay, Anderson and Cook41 Abnormal communications can also result from rupture of an acquired or congenital aneurysm of a sinus of Valsalva, infective endocarditis, aortic dissection, and traumatic or iatrogenic injury.
Two-dimensional assessment of the normal and congenitally malformed aortic root
Normal aortic root dimensions
Accurate imaging requires an adequate terminology. The virtual basal ring extends between the nadirs of the semilunar leaflets. Long-axis 2-dimensional imaging will hover between an off-center plane joining the nadirs of the right and noncoronary leaflets and a nonspecific center plane.Reference Mori, Izawa, Shimoyama and Tretter42,Reference Mori, Anderson and Tahara43 It is difficult to be sure of the true plane of the virtual basal ring itself without the use of 3-dimensional imaging and multiplanar reformatting.Reference Tretter, Izawa and Spicer4 Long-axis imaging, in the absence of such 3-dimensional imaging, often cuts the virtual basal ring in oblique fashion, with an increasing value achieved when moving toward a nonspecific center plane (Figure 6).Reference Tretter and Mori44 These caveats underscore the limitations of the continuity equation in assessing the dimensions of the oval virtual basal ring.
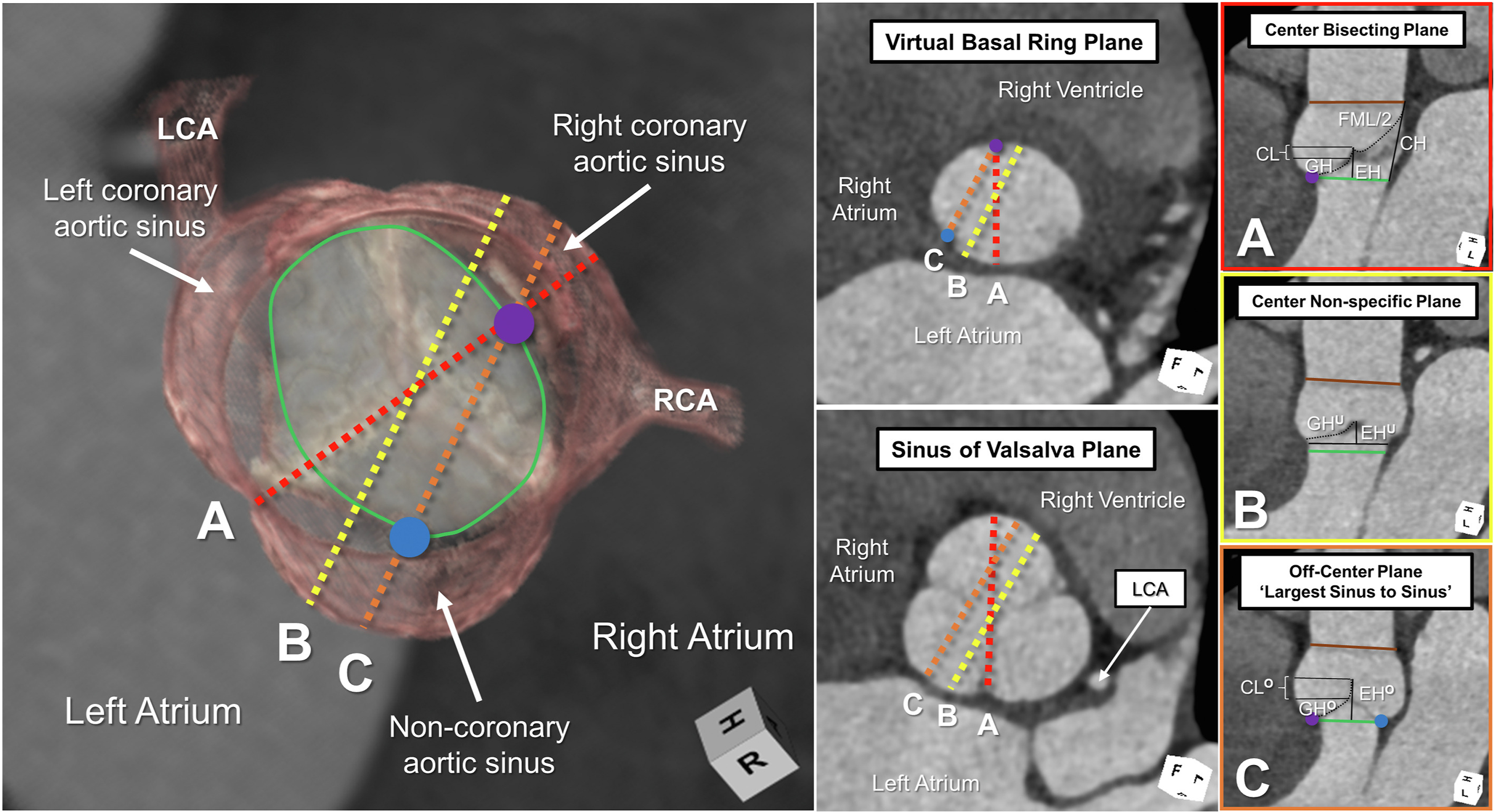
Figure 6. The left-side image demonstrates a 3-dimensional reconstruction of a normal aortic root in diastole, with the virtual basal ring marked with the green oval. The blue and purple circles mark the nadirs of the assessed leaflets. The middle panels demonstrate short-axis 2-dimensional planes at the virtual basal ring and aortic root. (A-C) The right-side panels are framed in the color of the corresponding colored dashed lines in the left-side panels. Only the center bisecting plane (A), with accurate marking of the virtual basal ring plane using multiplanar reformatting, permits precise measurement of the effective height (EH), coaptation length (CL), geometric height (GH), commissural height (CH), and free margin length (FML). Long-axis imaging of the aortic root, as obtained by 2-dimensional echocardiography, hovers between lines B and C, leading to underestimation (superscript U) and overestimation (superscript O) of these various metrics. The brown line represents the plane of the sinutubular junction. (LCA, left coronary artery; RCA, right coronary artery.)
Two-dimensional assessment of the sinuses by long-axis imaging provides a maximal dimension when moving from the off-center plane joining the nadirs of the visualized leaflets toward a nonspecific center plane. An apparent decrease in the coaptation length will be noted centrally (compare Figure 2 and Figure 6). This provides a clue that the cut plane is approaching the central zone of coaptation.Reference Tretter, Izawa and Spicer4,Reference De Kerchove, Momeni and Aphram8,Reference Mori, Anderson and Tahara43
Three separate methods have been proposed to measure the short axis of the root (Supplemental Figure 4). Of these measurements, 2 carry the name “cusp-to-cusp.” The original method is better described as “largest sinus-to-sinus” dimension. The more recent method provides a “center of sinus-to-center of sinus” measurement,Reference Tretter and Mori44 which correlates well with long-axis measurements.Reference Rodríguez-Palomares, Teixidó-Tura and Galuppo45 Only the “largest sinus-to-sinus” dimensions have normative values established in adults using magnetic resonance imaging.Reference Burman, Keegan and Kilner46 The “cusp-to-commissure” method, more accurately described as “center of sinus-to-opposite commissure,” does have normative adult values.Reference Tretter and Mori44 This method correlates strongly with 3-dimensional volumes.Reference Suzuki, Mori and Izawa7 No evidence currently supports the use of one method over the others, nor are we aware of any evidence to suggest the benefits of measuring “leading edge to leading edge” vs “inner edge to inner edge,” or using a specific time of the cardiac cycle. Institutional consistency is therefore paramount, with reporting provided of both the method used and the timing of the cardiac cycle.
Compared with the virtual basal ring, the sinutubular junction is relatively more circular. Its plane differs by approximately 5° to 10° relative to that of the virtual basal ring, with a lower tilt angle produced during ventricular systole.Reference Tretter, Spicer, Mori, Chikkabyrappa, Redington and Anderson6,Reference Izawa, Mori and Tretter17
Dimensions of the congenitally malformed aortic root
Assessing dimensions becomes increasingly complex when the root is congenitally malformed. In the functionally unileaflet and bileaflet roots, there is frequent significant asymmetry between the aortic leaflets and sinuses, potentially making transthoracic echocardiography inaccurate in this setting.Reference Vis, Rodríguez-Palomares and Teixidó-Tura47 This technique should maintain its central role in the serial follow-up, but there should be a lower threshold also for obtaining cross-sectional imaging to provide the most accurate measurements. The cross-sectional methods established for the trisinuate root are inappropriate for measuring the bisinuate root. Instead, it may be more appropriate to measure from center of sinus-to-center of sinus, with subsequent measurement from commissure-to-commissure.Reference Tretter and Mori44 Similarly, trisinuate methods should not be applied to the quadrisinuate root. All said, there are no normative values for comparison to these patients without a trisinuate root. In the congenitally malformed aortic root with hypoplasia of 1 or more interleaflet triangles, furthermore, it becomes challenging to define the plane of the sinutubular junction. In these rare variants, suggested methods thus far are guided by opinion only. When monitoring these patients, given the complexity of measuring the congenitally malformed aortic root, it may be helpful to measure cross-sectional areas or three-dimensional volumes.Reference Suzuki, Mori and Izawa7
Standardized assessment of aortic valvar dysfunction
Echocardiographic assessment of the degree of aortic valvar stenosis and regurgitation is well established, occasionally complimented by cardiac magnetic resonance imaging.Reference Baumgartner, Hung and Bermejo48 More recently, standard assessment of the aortic leaflets and their diastolic competency has been established using 3-dimensional imaging with multiplanar reformatting (Supplemental Table 1).Reference Tretter, Izawa and Spicer4,Reference Izawa, Mori and Tretter17,Reference Hagendorff, Evangelista, Fehske and Schäfers25 Such measurements have been used to guide a geometric approach toward surgical repair or preservation of the aortic valve.Reference Schäfers, Bierbach and Aicher49,Reference The50 They are commonly assessed in middiastole.Reference Izawa, Mori and Tretter17,Reference Hagendorff, Evangelista, Fehske and Schäfers25,Reference Mori, Izawa, Shimoyama and Tretter42 Accurate assessment, where a difference of a few millimeters becomes important, can only be achieved when using the center bisecting plane (Figure 6A, red dotted in the left-side panel), having accurately identified the plane of the virtual basal ring.Reference Izawa, Mori and Tretter17,Reference Mori, Izawa, Shimoyama and Tretter42
The dimensions of the leaflets are based on their geometric height,Reference Mori, Izawa, Shimoyama and Tretter42,Reference Schäfers, Bierbach and Aicher49,Reference Schäfers, Schmied, Marom and Aicher51 a curvilinear measurement taken within the extent of the root along the midline of the leaflet from its nadir at the virtual basal ring to the center point of its free margin edge, along with the free margin length, a curvilinear measure of the distance between the commissures along the edge of the leaflet.Reference Mori, Izawa, Shimoyama and Tretter42 The commissural height is the distance from the virtual basal ring along the long axis of an interleaflet triangle to the sinutubular junction. Comparison of these measurements becomes increasingly important in the congenitally malformed aortic root with 1 or more zones of fusion between the leaflets and with hypoplasia of the corresponding interleaflet triangles.
The coaptation length represents the linear extent of the segment of apposition involved at the central point of coaptation. When moving laterally from the center bisecting plane, the visualized coaptation length increases relative to the normal increased coaptation surface area along the lateral aspects of the zone of apposition (compare Figure 2 with Figure 6).Reference Tretter, Izawa and Spicer4,Reference De Kerchove, Momeni and Aphram8 Standardized assessments, obtained from the central bisecting plane, are obtained at the central point of coaptation.Reference Tretter, Izawa and Spicer4 The effective height is the linear measurement from the center of the virtual basal ring to the cephalad edge of the central segment of coaptation of the leaflets. Normative values have now been reported intraoperatively,Reference De Kerchove, Momeni and Aphram8,Reference Schäfers, Schmied, Marom and Aicher51 and more recently for adults using computed tomography.Reference Tretter, Izawa and Spicer4
Comment
After extensive discussions between members of the International Society for Nomenclature of Paediatric and Congenital Heart Disease, and other included experts relative to the assessment and management of the congenitally malformed aortic root, we offer here our suggestions for description of the congenitally malformed aortic root relative to the normal aortic root. The Nomenclature Society, constituted in 2005, produced the International Paediatric and Congenital Cardiac Code, which contains thousands of terms, each labeled with a 6-digit code. A “short list” of more than 350 items from this overall code has now been incorporated into the recently released 11th iteration of the International Classification of Diseases provided by the World Health Organization.Reference Jacobs, Franklin and Béland52 Our chosen terminologies of the aortic root are also consistent with those included in the most recent edition of the Terminologia Anatomica.53 We anticipate that the content of this review will serve further to amend both the existing International Code and the congenital heart section of the International Classification of Diseases 11th Revision. They will hopefully serve as the basis for ongoing and future clinical care and research across all cardiac specialties.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1047951123001233
Funding source
The authors have no funding sources to disclose.
Disclosure
The authors have no conflicts of interest to disclose.











