We present a case of subarachnoid hemorrhage (SAH) with diffuse spinal and posterior fossa involvement due to Eosinophilic Granulomatosis with Polyangiitis (EGPA), previously known as Churg–Strauss syndrome, presenting with mononeuritis multiplex. To our knowledge, this is the first case of EGPA complicated by both spinal and intracerebral SAH.
A 56-year-old lifelong asthmatic man presented to the emergency department with a sub-acute history of burning right foot pain ascending up his leg. The patient had a 1-year history of chronic cough for which he was taking inhaled steroids. There were no clear symptoms of pre-existing EGPA such as joint involvement, rashes, hemoptysis or nasal crusting or discharge. Family history was negative for autoimmune conditions. Initial neurological exam showed patchy sensory loss in both the upper and lower extremity as well as distal right leg weakness. Presenting labs were significant for an eosinophilia of 8.8, elevated erythrocyte sedimentation rate, and C-reactive protein as well as mildly elevated creatine kinase at 289. Urine showed proteinuria and blood but was not formally examined for active sediment. Further investigations performed shortly after admission showed a positive antineutrophil cytoplasmic antibodies (ANCA) profile with elevated myeloperoxidase antibody and staining predominately for perinuclear-ANCA. Extractable nuclear antigen panel was positive only for centromere B antibodies. Chest computed tomography (CT) scan showed diffuse airway abnormality with scattered mucous plugging and thickening of the bronchial walls. Nerve conduction studies of both lower limbs and the left upper limb were performed. The studies revealed decreased sensory nerve action potentials and compound motor action potentials with acute distal neurogenic changes, more severe in the right leg compared to the left. These findings were consistent with an asymmetric acute axonal motor and sensory neuropathy. This, in context of his history and examination, was in keeping with rapid progression of multiple mononeuropathies to the point of becoming confluent. The findings on the nerve conduction study along with the eosinophilia and changes on the chest CT were felt to be suggestive of EGPA. A right sural nerve biopsy with surrounding muscle was taken to confirm the diagnosis. The biopsy showed vasculitis with prominent eosinophilic inflammatory infiltrates, perivascular cuffing, and thrombosis. Treatment of suspected EGPA was initiated while the results of the nerve biopsy were still pending. Three doses of IV methylprednisone were administered followed by high-dose oral prednisone.
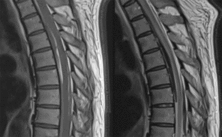
Twenty-four hours after the initiation of oral steroids, the patient experienced acute-onset chest pain with headache followed by decreased level of consciousness. At this time, the patient also had brief but marked hypertension with a systolic blood pressure measured as high as 220 mmHg. Non-contrast CT and CT angiogram head showed posterior fossa SAH centered around the pons with obstructive hydrocephalus secondary to intraventricular hemorrhage in the fourth ventricle (Figure 1). An external ventricular drain was inserted in the intensive care unit at that time. The patient’s level of consciousness fluctuated over the course of the following day. Brain MRI was then conducted. This redemonstrated SAH around the right brain stem extending to the level of C2–C3 ventrally as well as moderate volume intraventricular hemorrhage and accompanying obstructive hydrocephalus. Interestingly, increased spinal T2 signal was visualized between T3 and T5 on the cervical spine MRI. This prompted dedicated spine imaging which showed posterior fossa SAH extending through the thoracic spine. There was also an area of thoracic cord thickening, central cord hyperintensity, and suspicion of cord compression centered at the level of T6 which extended between T3 and T8 (Figure 2). Spinal angiogram was normal and this was felt to be secondary to a possible subtle subdural component of the bleed causing cord compression and edema. Cerebrospinal fluid (CSF) analysis performed showed an elevated CSF protein at 1.22 but normal white count.

Figure 1: Axial unenhanced computed tomography of the head showing large subarachnoid hemorrhage centered around the right brain stem in the perimesencephalic cistern as well as hemorrhage in the fourth ventricle and resulting obstructive hydrocephalus with enlargement of the lateral ventricles bilaterally.

Figure 2: Mid sagittal T1 and T2 weighted thoracic spine images showing SAH and increased signal in the thoracic cord centered around the level of T6.
SAH is a rare complication of EGPA.Reference Matsuda, Yoshida and Fujiki1 In a retrospective review of patients with EGPA who were underwent long-term follow-up, approximately 8% had central nervous system (CNS) involvement.Reference Guillevin, Cohen, Gayraud, Lhote, Jarrousse and Casassus2 The majority of these cases were stroke (75%), but the type of stroke was not further classified. More recent publications have identified that cerebral SAH hemorrhage is more common whereas spinal SAH is relatively rare. A review of patients with EGPA and CNS involvement concluded that 57% of patients who underwent CT or MRI had radiological evidence of cerebral SAH while only 1% spinal cord involvement.Reference André, Cottin and Saraux3 A 2016 review by Decker et al. found only four reported cases of EGPA with spinal cord involvement in the literature.Reference Decker, Emery, Smyth, Lu, Lacson and Yacyshyn4 Only one of these four cases had spinal cord SAH and this case did not have cerebral involvement.Reference Diamanti, Berzero and Bini5
The purported mechanism of SAH in EGPA is unclear but has been proposed to involve a combination of medium vessel vasculitis and eosinophil-mediated injury.Reference André, Cottin and Saraux3 To our knowledge, this is the first case of simultaneous spinal and cerebral SAH in a patient with EGPA. The hemorrhages were discontinuous arguing against the spinal SAH simply being an extension of the cerebral SAH. This patient unfortunately had a poor outcome despite early initiation of steroid therapy. This highlights the potential for serious neurologic sequelae in EGPA even with early identification and treatment. Furthermore, while EGPA remains a very uncommon cause of cerebral SAH, vasculitidities such as EGPA have been reported in one case series as the cause of up to 25% of spontaneous spinal SAH.Reference Yost and Rabinstein6 In the absence of other clear causes such as trauma, vascular abnormalities, or hypertension, an underlying vasculitis should be considered in spontaneous spinal SAH.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
CS drafted the manuscript. CH collected the data and revised the manuscript.