Introduction
Parkinson disease (PD) is the second most common neurodegenerative disease worldwide and is growing more rapidly than Alzheimer’s disease.Reference Connolly and Lang1,Reference Abajobir and Abate2 Parkinsonism is an umbrella term that includes PD and other types of parkinsonisms. While the majority of cases of parkinsonism assessed in movement disorders clinics are due to PD, some are caused by secondary parkinsonism (drug-induced, vascular) and atypical parkinsonisms.Reference Roberts-South, Hall and Jog3 A subset of patients referred to such clinics may be diagnosed with other entities such as essential tremor as well. The atypical parkinsonisms, including multiple systems atrophy, progressive supranuclear palsy, corticobasal degeneration, and dementia with Lewy Bodies, have more rapid progression with shorter life expectancies compared to PD.Reference Grimes, Fitzpatrick and Gordon4 The Brain Disorders in Ontario Report (2015) documented that 82% of people with parkinsonism (PWP) were 65 years and older.Reference Ng, Maxwell and Yates5 The number of older Canadians with parkinsonism is expected to more than double from 71,500 to 148,800 by 2031.6
Most literature on parkinsonism has focused on people with PD (PWPD) due to the much higher prevalence compared to other parkinsonisms. Studies have found high rates of hospital admission in up to 33% of PWPD,Reference Hassan, Wu and Schmidt7–Reference Shahgholi, De Jesus and Wu9 with 51% readmission within 1 year.Reference Hassan, Wu and Schmidt7 PWPD also experience prolonged length of stay, complications, and functional decline during hospitalization.Reference Guttman, Slaughter, Theriault, DeBoer and David Naylor10–Reference Muzerengi, Herd, Rick and Clarke14 Reasons for hospitalization include motor complications, psychiatric symptoms, autonomic dysfunction, and side effects of antiparkinsonian drugs amongst others.Reference Klein, Prokhorov, Miniovitz, Dobronevsky and Rabey12,Reference Factor and Molho13,Reference Temlett and Thompson15 Hospital readmissions correlate with longer disease duration, co-morbidities, cognitive impairment, caregiver stress and non-motor symptoms (NMS).Reference Hassan, Wu and Schmidt7,Reference Shahgholi, De Jesus and Wu9,Reference Klein, Prokhorov, Miniovitz, Dobronevsky and Rabey12
Older PWPD, compared to younger ones, are at higher risk for repeat ED visits and hospitalization with longer lengths of stay,Reference Woodford and Walker11 as well as admission to long-term care after discharge.Reference Woodford and Walker11,Reference Klein, Prokhorov, Miniovitz, Dobronevsky and Rabey12
Several studies have suggested ways to potentially reduce hospitalization rates: frequent neurologist visits, better medication adherence/optimization, and the use of interdisciplinary services.Reference Shahgholi, De Jesus and Wu9,Reference Muzerengi, Herd, Rick and Clarke14 However, only one study found a reduction in hospitalization from 40 to 18 per year by endorsing an “open-door” policy to allow urgent clinic visits.Reference Klein, Prokhorov, Miniovitz, Dobronevsky and Rabey12
NMS, including neuropsychiatric (dementia, psychosis, depression, anxiety, and sleep disorders) and autonomic (orthostatic hypotension, constipation, urinary symptoms, dysphagia, temperature dysregulation) features become increasingly prevalent in advanced PD,Reference Lim and Lang16 and become the dominant reason for declining quality of life and function.Reference Poewe17–Reference Weintraub, Moberg, Duda, Katz and Stern19 The complexity of PD in older patients is recognized in the literature as a “Geriatric Syndrome”,Reference Lauretani, Maggio, Silvestrini, Nardelli, Saccavini and Ceda20 associated with more rapid progression, higher rates of NMS, higher comorbidities and polypharmacy, and more significant decline in function and quality of life.Reference Schrag, Jahanshahi and Quinn21 The high levels of frailty in PWPD, especially in older age, have also been documented recently in the literature.Reference Peball, Mahlknecht and Werkmann22,Reference Smith, Brennan, Gaunt, Ben-Shlomo and Henderson23 Comprehensive Geriatrics Assessment (CGA) and management have been shown to improve independence and survival for frail older people with Geriatric Syndromes and multiple medical conditions.Reference Lauretani, Maggio, Silvestrini, Nardelli, Saccavini and Ceda20,Reference Lauretani24–Reference Kalsi, Babic-Illman and Ross28 CGA is an interdisciplinary assessment of multiple domains of health: medical & physical conditions, medication review, nutrition, cognition, mood, activities of daily living, social support, and home safety. It generates problem lists and integrated, patient-focused treatment plans to provide goal-driven interventions, rehabilitation, and long-term planning.Reference Ellis, Gardner and Tsiachristas25–Reference Conroy, Stevens, Parker and Gladman27,Reference Welsh, Gordon and Gladman29
In recognition of the high volume and care needs of older PWP in our community, North York General Hospital (NYGH) Specialized Geriatric Services (SGS) established the Geriatrics Clinic for Parkinson’s in 2007 to provide care to older PWP using a CGA model. NYGH is a 420-bed community teaching hospital serving over 100,000 ED visits per year.30 It is located in a catchment area with relative high prevalence of parkinsonism and aged population.Reference Ng, Maxwell and Yates5 Patients in this clinic may get referred to other parts of SGS, such as the Geriatrics Day Hospital (an outpatient assessment and rehabilitation program) and Parkinson’s Education Program. The interprofessional team from the Day Hospital and Parkinson’s Education Program received the Allied health team training from the National Parkinson’s Foundation (NPF) in order to serve our PWP.31 Referrals to the clinic are received from family physicians, community neurologists, movement disorders neurologists, psychiatrists, and hospital-based physicians. Information about our service and an introductory video are available on our hospital website.32
Since 2007, our clinic was run by two core members: a Care of the Elderly physician with additional training in movement disorders at a tertiary care Movement Disorders Center, and a Board Certified Geriatric Pharmacist (BCGP)33 with extra training through American Society of Consultant Pharmacists and the Allied Team training by NPF.31 The clinic physician attended on the inpatient Geriatrics service and the pharmacist served in the Day Hospital and the Parkinson’s Education Program, which enhanced continuity of care.
The clinic aimed to provide ongoing support to patients and caregivers to prevent adverse clinical outcomes. Thus, a telephone intervention (TI) service, staffed by the clinic pharmacist, was established to provide timely advice between biannual clinic visits. Given the paucity of documented ED visit or hospitalization prevention strategies for older PWP/PWPD, we investigated the impact of our TI service on potential ED visits.
Objectives
We aimed to investigate (1) whether a pharmacist-led TI service in the Geriatrics Clinic for Parkinson’s (hereafter “the clinic”) could avert ED visits for older persons with parkinsonism (PWP) attending the clinic, (2) the reasons for calls, (3) the characteristics of patients associated with frequent calls (3 or more) and “crisis calls” (calls with intention to visit ED), and (4) user satisfaction.
Methods
We used a prospective observational cohort design to record all calls over a 12 month study period from January 1, 2016 to December 30, 2016. The Human Subjects Research Ethics Board of NYGH approved the study protocol (NYGH REB # 14-0004).
Our pharmacist-run TI service was available Monday to Friday from 9 am to 5 pm to allow all patients enrolled in the clinic and their caregivers timely access to advice by returning phone calls within one business day. No patients were excluded based on any characteristic. At the initial clinic appointment, the pharmacist met with patients and caregivers to document all medical conditions (based on available medical records and clinical history) and current medications in a free-text spreadsheet, which she updated at every follow up visit. PWP and caregivers were routinely instructed to call the pharmacist if issues should arise before the next appointment. Cognitive screening using the Mini Mental State Exam (MMSE) was done at the initial visit, along with Unified Parkinson Disease Rating ScaleReference Fahn34 (UPDRS) and other applicable scales. MMSE was conducted in the patient’s first language if s/he was not fluent in English through translation by caregiver/family or available staff. Patients were seen in clinic biannually for UPDRS part III (motor exam), NMS screening, and CGA with standardized tools to assess cognition, mood, etc. based on symptoms reported. The physician dictated detailed clinic notes as per usual geriatrics standards at each visit.
Data extracted from the clinic charts included patient demographics (at time of first call), number of comorbidities (defined as chronic medical conditions)Reference Guralnik35 and medications, diagnosis, duration of disease, most recent UPDRS Part III score, most recent MMSE score, and the presence of psychiatric morbidity (depression and/or anxiety) and/or dementia.
Using a collection tool (Appendix 1 in Supplementary material), the pharmacist documented who initiated the call and the reason for each call, intervention provided, call duration (defined as the actual duration of phone call, excluding other clinical activities), and outcome. In case of especially challenging clinical issues or if a prescription change was required, she would contact the clinic physician with a suggested intervention, for consultation and verbal prescription by telephone or secure email.
Each caller was asked at the time of the initial call with a specific question, “Would you bring (patient’s name) to ED if the problem was not improved or resolved by this phone call?”. Calls with intention to visit ED were classified as “crisis calls”. Within 1 week of each crisis call, the pharmacist provided telephone follow up, to see if the issues were resolved, and if patients visited ED. The pharmacist reviewed patients’ electronic medical records (EMR) at NYGH to verify ED visit or lack thereof. Outcome was documented in the form (Appendix 1 in Supplementary material) which was scanned into the electronic medical record. A research assistant contacted all callers within 3 weeks for an anonymous survey to document level of satisfaction and confidence in the TI service (Appendix 2 in Supplementary material).
In order to analyze if there is any association between patient characteristics and frequent calls or crisis calls, patients were labeled as “crisis callers” if they/their caregivers made at least one crisis call. Similarly, patients were labeled as “frequent callers” if three or more calls were made concerning them. Statistical analyses using logistic regression were carried out using SAS v.9.4 software.
Results
Sources of Calls and Patients’ Characteristics
We received 337 calls regarding 114 patients. Most calls were by spouse carers (37%), followed by adult children (29%) and other caregivers (15%). Patients only initiated 14% of the calls. Overall, 5% were from other health care professionals involved in the patients’ care. Average duration of the calls was 19 min. Patient characteristics are presented in Table 1.
Table 1: Patient characteristics

Reasons and Outcomes of All Calls
Out of 337 calls, the most common reasons for calls were NMS (94 calls or 27.9%), followed by adverse drug effects (ADE) (72 calls or 21.4%) and motor symptoms (MS) (62 calls or 18.4 %). Other less common reasons included request for medication refills, drug interactions, and other medical issues unrelated to PD (Figure 1 and Table 2).
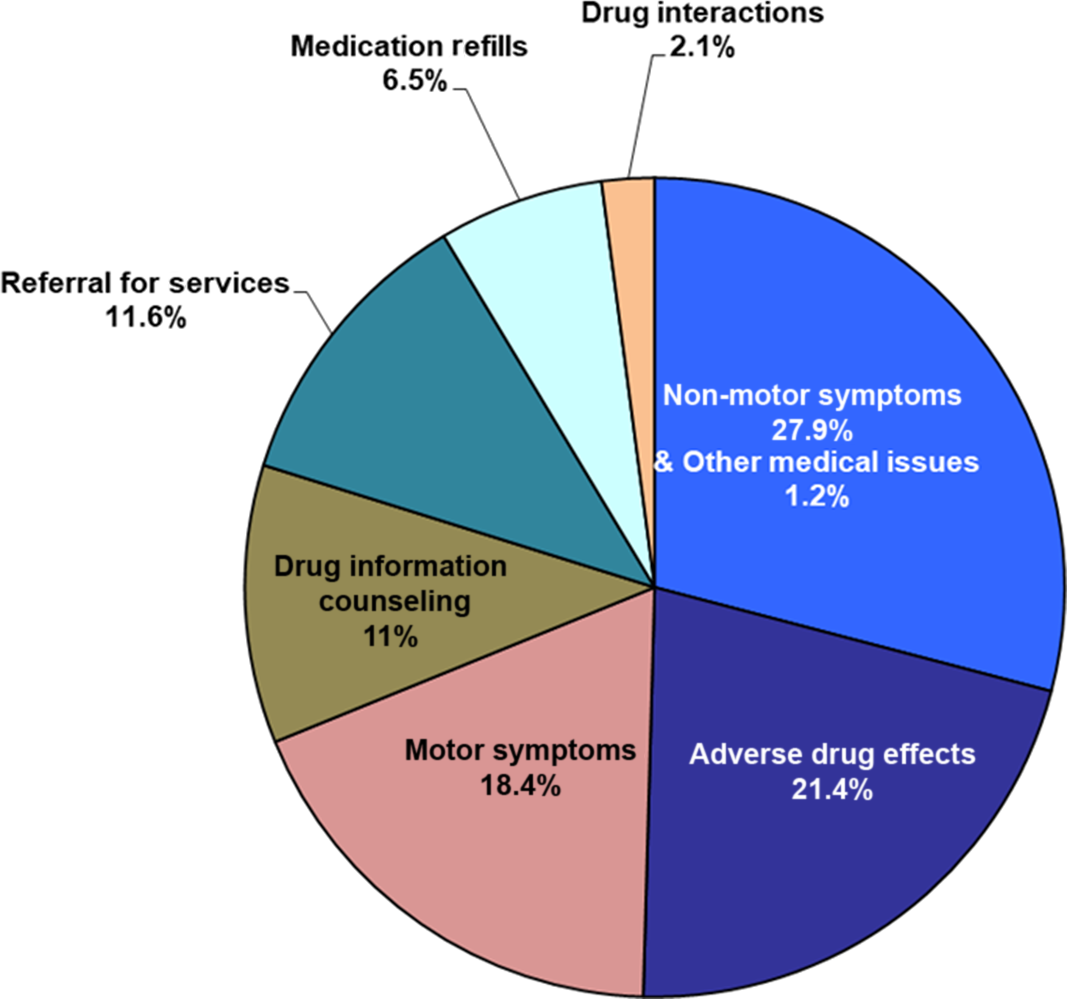
Figure 1: Reasons for 337 calls.
Table 2: Reasons for calls and outcomes of total calls and crisis calls

In total, 97.9% (330/337) of all calls were resolved without ED visit, and 92.9% (313/337 calls) were resolved through telephone advice only. Overall, 69.7% of calls required medication changes, 3.0% (10/337) required urgent appointment with the family physician or other specialist physicians, 2.1% (7/337) required urgent appointment with our clinic, and only 2.1% (7/337) presented to ED (Table 2).
Reasons and Outcomes for Crisis Calls
About 1 in every four calls (24.3%) were classified as “crisis calls” (total = 82) based on intention to visit ED. In total, 46% of the 114 patients (or their caregivers) initiated at least one “crisis call”. NMS accounted for the largest proportion of crisis calls at 39% (32/82 calls), followed by ADE at 29.3% (24/82 calls), while MS only accounted for 18.3% (15/82 calls). Of note, all patients enrolled in the clinic had family physicians as that was a criterion for acceptance of initial referral to our Geriatrics Service.
A total of 25 crisis calls (30.5%) were associated with psychosis. These cases were classified either as NMS from PD or as ADE depending on whether a reduction or discontinuation of offending medication(s) resulted in resolution of psychosis. Psychosis was the most common NMS (15 of 32 cases) and ADE (10 of 24 cases) leading to “crisis calls” (Tables 2 and 3). Three calls were due to drug interactions (Descriptions in Table 3). Three calls were for urgent referrals (two for palliative care and one for wound care). Four calls were for other medical issues presumably unrelated to PD (fever; congestive heart failure (CHF); lethargy from dehydration; and urinary symptoms with confusion from suspected urinary tract infection (UTI); of which all except UTI were advised to go to ED), and one call was for drug information regarding the use of rectal levodopa at end stage PD with NPO status.
Table 3: Distribution of non-motor symptoms, adverse drug effects, drug interactions reported from crisis calls

Only six patients from these 82 crisis calls actually visited ED when followed up within a week. Of note, the pharmacist advised these individuals to go to ED based on clinical judgment during the initial call. The diagnoses from the ED visits were: dehydration with delirium (1), fever due to septicemia (1), end-stage CHF (1), anxiety with insomnia (1), and falls (2). After TI, the culprit issues were resolved at time of follow up, and patients did not visit ED in 76 of 82 crisis calls (93%) (Table 2).
One non-crisis call reported recurrent symptoms of heartburn with prior history of Gastroesophageal reflux disease. The patient was advised to resume pantoprazole and go to ED if symptoms did not resolve. The patient attended ED and was admitted to hospital for coronary angiography.
Potential Association of Patient Characteristics with Crisis Calls or Frequent Calls
No patient characteristics had a statistically significant association with crisis calls. Characteristics with the highest point estimates were: high polypharmacy (11+ medications; OR of 1.71, CI 0.81–3.60, p-value = 0.16), psychiatric morbidity (anxiety and/or depression; OR 1.89, CI 0.84–4.26, p-value = 0.12), and dementia (OR 1.67, CI 0.79–3.54, p-value = 0.17) (Table 4).
Table 4: Individual patient characteristics with respect to crisis calls and frequent calls

Out of 114 callers, 45 (39.4%) called three or more times during the study period, classified as “frequent callers”. Odds ratio point estimates are highest between high frequency of calls and psychiatric morbidity (OR 1.56, CI 0.69–3.56, p-value = 0.29), and dementia (OR = 1.41, CI 0.67–3.00, p-value = 0.37), although these associations were not statistically significant (Table 4).
User Satisfaction and Confidence in Service
In total, 97.4% of the 114 callers agreed or strongly agreed that they were satisfied with our TI service (Likert scale 4 or 5 out of 5), while 92.1% of callers agreed or strongly agreed that they were sufficiently confident in the service that they would always contact the clinic first before going to ED (Likert scale of 4 or 5 out of 5).
Discussion
Our study is the first TI study focusing on a population of older PWP in a Specialized Geriatrics setting, with a focus on prevention of ED visits. Our sample was largely composed of patients with idiopathic PD at 76%, followed by atypical parkinsonism. This proportion, which depends largely on referral patterns, appears to be typical of most Movement Disorder Centers. Reference Fahn34,Reference Guralnik35 Thus, our findings may potentially be generalizable to the sector of older patients within these settings.
While literature has shown that PWPD and their caregivers would benefit from more support for symptom management, caregiver stress, wellness strategies, and future planning, there is still lack of widespread availability of such support. Reference Lageman, Mickens and Cash36 TI services Reference Roberts-South, Hall and Jog3 and utilization of telemedicine have been proposed to address some of these unmet needs. Reference Dorsey, Venkataraman and Grana37–Reference Pretzer-Aboff and Prettyman41 A review of the literature revealed three studies in TI in Movement Disorders Centers. Reference Roberts-South, Hall and Jog3,Reference Liu, Boxhorn and Klufas42,Reference Adam, Ferrara, Aguilar Tabora, Nashatizadeh, Negoita and Jankovic43 One study noted that neuropsychiatric symptoms like anxiety and sleep disorders predicted frequent phone calls in non-demented PWPD. Reference Liu, Boxhorn and Klufas42 Another study of TI staffed by fellows and neurologists highlighted that patients with PD and atypical parkinsonism contributed disproportionately, compared to other movement disorders, to the phone calls. Reference Adam, Ferrara, Aguilar Tabora, Nashatizadeh, Negoita and Jankovic43 The study on a nurse-run TI service reported on the reasons for calls and the outcomes and emphasized the complexity of medical issues faced by PWP and the need for more widespread availability of TI support. Reference Roberts-South, Hall and Jog3
Reasons for All Calls and Overall ED Utilization Rate
Similar to the other two studies, the majority of our calls were related to worsening of symptoms and issues related to medications. Reference Roberts-South, Hall and Jog3,Reference Adam, Ferrara, Aguilar Tabora, Nashatizadeh, Negoita and Jankovic43 Top reasons for all 337 calls in our study were NMS (28%), followed by medication issues (24%) which included ADE (21%) and drug interactions (3%), and lastly MS (18%). Our findings contrast with the TI study in a younger group of PWP (age 67.9), where MS was the most common reason (27%), followed by medication issues (22.4%), and NMS (10.4%) despite a similar duration of disease (9.8 years in their study vs. 9.5 years in ours). Reference Roberts-South, Hall and Jog3 This highlights the much more dominant role of NMS in older PWP compared to younger PWP in terms of seeking clinical support. This is consistent with literature which has noted that older age of onset of PD may be associated with relatively more NMS compared to younger age of onset. Reference Mendonça, Lampreia, Miguel, Caetano, Barbosa and Bugalho44,Reference Zhang, Yu and Guo45
Our study found that most (70%) of the calls required medication management/adjustment, in contrast to lower proportions of 42.5% in Adam et al.’s study and 35% in Roberts-South et al.’s study. Reference Roberts-South, Hall and Jog3,Reference Adam, Ferrara, Aguilar Tabora, Nashatizadeh, Negoita and Jankovic43 This is expected when caring for an older population, given the volumes of literature documenting the high risk of ADE, polypharmacy and the value of medication management in older individuals. Reference Onder, Lattanzio and Battaglia46–Reference McLean, Hindle, Guthrie and Mercer51 This further suggests that medication management is an essential component in the care of older PWP and should be included in care planning for this population. In our study, earlier clinic appointment was necessary for issues that could not be resolved over the phone for 2% of patients, compared to 5.8% in Roberts-South et al.’s study and 2.5% in Adam et al.’s study. Reference Roberts-South, Hall and Jog3,Reference Adam, Ferrara, Aguilar Tabora, Nashatizadeh, Negoita and Jankovic43 Thus, even for an older population of PWP, with the higher inherent risks of poor health outcomes, TI was able to resolve issues and divert the need for earlier clinic appointments, which is an important consideration given limited resources.
Despite having an older patient population, we had a similarly low overall ED utilization rate of 2% amongst all calls vs. 2.56% in the nurse-led study and 1.26% in physician-led study, although the other studies did not specifically study ED visit intent or aversion. Reference Roberts-South, Hall and Jog3,Reference Adam, Ferrara, Aguilar Tabora, Nashatizadeh, Negoita and Jankovic43
Crisis Calls and ED Visit Aversion
In order to determine if our TI averted ED visits, we prospectively asked the callers regarding whether they intended to visit ED, and classified those as “crisis calls”. The prospective question at the time of initial phone call prevented recall bias, reflected the caller’s perspective of what constituted a “crisis”, and documented their original plan of action, rather than our clinical opinion. We feel this was crucial in the methodology and would reflect the burden experienced by the patients/caregivers more accurately.
About one out of every four calls was specified by callers as “crisis calls”. Reasons for these 82 crisis calls were NMS (39%), medication issues 32.7% (including ADE (29%) and drug interactions (3.7%)), and MS (18%). The proportion of NMS and medication issues was even higher among crisis calls compared to all calls, accounting for almost three quarters of crisis calls. In total, 92.7% of these callers who indicated intention to visit ED did not end up going to ED after TI. Although there was no control group, our data suggests that our TI was successful in aversion of potential ED visits. We suspect that spontaneous resolution of the issues leading to “crisis calls” (see Table 3) would be very unlikely without appropriate clinical intervention in this older population of PWP.
According to the Canadian Institute for Health Information, one in five ED visits in Canada could have been prevented with better care and support from medical clinics. 52 Ontario’s Telehealth service advised 25% of all callers in 2018/19 to go to ED. Reference Basky53 The Director of one Telehealth Service acknowledged that the protocols used by telehealth nurses to assess callers err on the side of caution because assessment over the phone is limited. Reference Basky53 In contrast, TI run by our clinical pharmacist, who was familiar with the patients’ medical conditions and medications, only advised 7.3% of callers with “crisis” issues to go to ED. This highlights the significant potential cost savings for the public or single payer health care system if a similar clinician-run TI service was broadly integrated into longitudinal care for the older PWP population.
Implications for Care Planning for Older PWP
Given the high risk of hospitalization and associated poor outcomes for older PWP, prevention of ED visit and hospitalization is essential and must be part of care planning. Multiple studies have described strategies such as frequent neurologist visits, medication management, interdisciplinary approach, and addressing caregiver burden to prevent hospitalization. Reference Hassan, Wu and Schmidt7,Reference Shahgholi, De Jesus and Wu9,Reference Muzerengi, Herd, Rick and Clarke14 The most effective strategy to date cited 50% reduction of admission using an “open door policy” in a younger population. Reference Klein, Prokhorov, Miniovitz, Dobronevsky and Rabey12
Our study data suggests that 92.7% of potential ED visits were averted by TI in our older population with high hospitalization risk due to comorbidity and polypharmacy. Reference Roberts-South, Hall and Jog3,Reference Ng, Maxwell and Yates5,Reference Shahgholi, De Jesus and Wu9
Telephone-based interventions may form one part of a larger interdisciplinary approach in caring for older PWP, which also includes CGA, medication management, and enhanced caregiver support. These may help address the unique needs and characteristics of this population including:
-
1. higher NMS and medication issues
-
2. heightened importance of the caregiver, who initiated 81% of the calls in our study.
Comprehensive Geriatrics Assessment for Older PWP
Our study highlighted the predominance of NMS in “crisis calls” and all calls in our older PWP population. This is consistent with literature. Reference Zhang, Yu and Guo45,Reference Hu, Ou and Liu54 In particular, the impact of neuropsychiatric disturbances, cognitive impairment on quality of life, and caregiver burden may exceed that of MS. Reference Barone, Antonini and Colosimo55–Reference Grün, Pieri, Vaillant and Diederich58 Neuropsychiatric and cognitive issues were reported in over half of our patient population. Although we did not specifically measure frailty, the high proportion of NMS and advanced age of our patients would suggest a high co-occurrence of frailty based on existing associations from the literature. Reference Peball, Mahlknecht and Werkmann22,Reference Smith, Brennan, Gaunt, Ben-Shlomo and Henderson23,Reference Tan, Hew and Lim59 Since CGA is the recognized standard in caring for older individuals with frailty in multiple medical conditions, Reference Ellis, Gardner and Tsiachristas25–Reference Welsh, Gordon and Gladman29 it may also be an appropriate and effective approach in caring for older PWP.
An interdisciplinary, comprehensive treatment plan with goals of care is formulated through CGA, and serves as guidance for the clinic pharmacist in provision of TI advice. The CGA approach may be one way to operationalize the recommendations from the literature and PD guidelines: PWPD should have a comprehensive, personalized care plan agreed between the patient, their family/caregivers, and the interdisciplinary team of care providers. Reference Grimes, Fitzpatrick and Gordon4,Reference Okun60–Reference Rogers, Davies, Pink and Cooper62
More widespread adoption of a CGA approach for older PWP, in a collaborative model with movement disorders specialists, is an area of opportunity for the medical community to potentially improve our care for this population, and further research on this topic would be important.
Since the completion of this study, our clinic has expanded to include a nurse and a neurologist to broaden the scope of interprofessional care provided for older PWP.
Expert-Led Medication Management
This is the first pharmacist-led TI study in a Parkinson’s Clinic. One of the main reasons for crisis calls was medication issues (drug interaction and ADEs – Table 3), such as psychosis from antiparkinsonian medications, falls from cumulative hypotensive effects of drugs like beta blockers, alpha blockers (often prescribed for urinary issues in men), dopaminergic medications, and antidepressants.
The relation of increased ADE risk to age is well described and is one of the top reasons for hospitalization of older adults. Reference Onder, Lattanzio and Battaglia46,Reference Lavan and Gallagher49,Reference Routledge, O’Mahony and Woodhouse63,Reference Beijer and De Blaey64 The World Health Organization highlights polypharmacy as one of the key focus areas of its Global Patient Safety Challenge Reference Klein, Prokhorov, Miniovitz, Dobronevsky and Rabey12,Reference Schrag, Jahanshahi and Quinn21 and a leading problem in elderly patients in general and those with PD. Reference McLean, Hindle, Guthrie and Mercer51,Reference Müller-Rebstein, Trenkwalder, Ebentheuer, Oertel, Culmsee and Höglinger65 Simplifying the drug regimen has been recommended in a large NPF hospitalization study of PWP. Reference Shahgholi, De Jesus and Wu9 The CGA approach has proven to be beneficial in improving not just polypharmacy, but the appropriateness of medications being taken by older adults. Reference Unutmaz, Soysal, Tuven and Isik50
Given the high frequency of call issues that required medication management (69.7%), the optimal individual to fill the TI role is a professional with extensive medication experience in parkinsonism and other chronic medical conditions. Use of a geriatrics pharmacist, as was done in our study, is one avenue to address this clear need. Healthcare professionals with similar experience (e.g. geriatricians, movement disorders specialists, geriatric nurses with training in Parkinsonism) could also potentially fill this role.
The required personnel time of the pharmacist to run TI in our case, to serve all of our enrolled clinic patients (170 in December 2016), with 114 patients/caregivers using the service, 19 min of on the phone time per call, and about 20 min for clinical activities off the phone per call, would be around 220 h per year, which is about 1 h per business day (without considering time spent on follow up). The time requirement has proportionally increased over time with our clinic enrollment. Given the time commitment of 1 h/day for the TI role, an allied health professional such as a geriatrics pharmacist or nurse would be a more practical choice than a physician from a resource perspective.
Enhanced Caregiver Support Through Telephone Intervention
The majority (114 or 67%) of our 170 enrolled clinic patients (or their caregivers) (as of December 2016) utilized the TI service compared to 43% in a previous study of younger patients. Reference Roberts-South, Hall and Jog3 The majority of the calls were initiated by caregivers (81%) rather than patients, in contrast to only 33% in the younger population. Reference Roberts-South, Hall and Jog3 This suggests that when caring for an older population of PWP, the role of caregivers, caregiver stress and support are much more important compared to that in a younger population. This factor must be recognized and reflected in service design.
Neuropsychiatric complications, including psychosis, depression and dementia, which are more common in older PWP, are known in the literature to be correlated with increased caregiver stress Reference Martinez-Martin, Rodriguez-Blazquez and Forjaz57,Reference Schrag, Hovris, Morley, Quinn and Jahanshahi66 and hospitalization. Reference Shahgholi, De Jesus and Wu9 Our study found a total of 32 cases of psychosis (almost 10% of 337 calls), of which 25 led to crisis calls (accounting for 30% of crisis calls). One study which explored caregiver burden in Lewy body dementia (LBD) (including Parkinson disease dementia (PDD) and Dementia with Lewy Bodies (DLB)) found that almost 2/3 (64%) of the caregivers experienced a crisis situation in the past year and 73% of those ended up seeking help in ED. Reference Galvin, Duda, Kaufer, Lippa, Taylor and Zarit67 Our population had a high prevalence of psychiatric morbidity, dementia (majority being PDD), and psychosis, which would suggest that our caregivers likely experienced high caregiver stress based on known associations. Indeed, almost half of our 114 users made a crisis call in 2016. Provision of accessible and practical support through TI to such stressed caregivers may help prevent escalation of crises and ED visits.
Literature and Guidelines have emphasized the importance of addressing caregiver burden in PD and LBD. Reference Grimes, Fitzpatrick and Gordon4,Reference Shahgholi, De Jesus and Wu8,Reference Rogers, Davies, Pink and Cooper62,Reference Galvin, Duda, Kaufer, Lippa, Taylor and Zarit67 In addition to clinical characteristics discussed above, literature has noted that fragmentation of care, multiple visits to different specialists, inadequate communication, poor transitions of care, and lack of a single trusted point of contact are frequent complaints of older patients and are a significant source of caregiver stress. Reference Taylor and Quesnel-Vallée68
TI by the clinic pharmacist with whom the patients and caregivers have a therapeutic relationship may have served as a form of enhanced caregiver support and a trusted point of contact. The prompt response (within one business day) and individualized attention for an average of 19 min per call may have contributed to building confidence and trust. It should be noted that the average call duration in our study was much longer than that reported previously (6.6 min) in a fellow-run TI service. Reference Adam, Ferrara, Aguilar Tabora, Nashatizadeh, Negoita and Jankovic43 Multiple factors may account for the difference in duration of calls between the two studies. A post-TI anonymous survey by our research assistant (who was not part of the clinical staff, to reduce bias) found that 97.4% of our callers agreed or strongly agreed that they were satisfied with the TI, while 92.1% agreed or strongly agreed that they were sufficiently confident in the service, to always contact the clinic before going to ED. The trust and confidence in a familiar expert clinician, in contrast to a hotline like Provincial Telehealth service, Reference Basky53 may be an important factor in providing effective TI to prevent ED visits.
Limitations
The main limitation of our study was the lack of a control group. We did not include a control for two reasons: it was an exploratory study; and it would have been unfeasible to exclude a portion of patients in our clinic from the TI service when we have run the clinic since 2007 with TI in place. We were not able to determine exactly which aspects of our approach resulted in the success in aversion of ED visits, whether it was the CGA approach, the use of a pharmacist, the 24 h turnaround, or an established therapeutic relationship. While the approach could potentially be generalizable to similar settings in Canada, it is unclear whether it would be generalizable to other countries and other payer systems. The total number of calls, and specifically, numbers of crisis callers and frequent callers, may have been too small to detect significant associations with patients’ characteristics. Due to privacy regulations, we were unable to access the Provincial EMR database to verify if patients visited other ED’s than the one at our hospital. The 1 week follow up calls were hopefully sufficient to reduce recall bias.
Conclusions
TI delivered by a Geriatric pharmacist within a Geriatrics Clinic for Parkinson’s averted 93% of potential ED visits in our older population who had high hospitalization risk due to comorbidity and polypharmacy.Reference Roberts-South, Hall and Jog3,Reference Ng, Maxwell and Yates5,Reference Shahgholi, De Jesus and Wu9 In addition, the service was recognized as highly acceptable and trusted by the callers, who were mostly caregivers. TI, when delivered within a CGA framework, including medication management by a board certified geriatric pharmacist, may be particularly suited to the needs of older PWP and their caregivers.
This approach or some aspects of it could be further explored as a collaborative model in movement disorders settings caring for older PWP, and has the potential for substantial cost savings in a single payer or public health care system through reducing ED utilization and hospitalization. However, as countries with different payment systems move towards “value-based reimbursement” (or “pay for performance”), this kind of model may be increasingly relevant.Reference Cherian and Rakowski69,70
Future research could compare TI offered in a CGA setting by a pharmacist, vs. other health professionals, and/or in other clinical settings/models. This would help determine the essential factors and best practices for ED visit and hospitalization prevention for older PWP. It would be important to determine the health economics implications of the intervention. More studies on the characteristics of older PWP served in different clinical settings, including Movement Disorders Centers, Geriatrics Clinics, and general neurology clinics would help determine whether certain subsets of patients are more frail and at higher risk of poor outcomes, and thus should receive more targeted interventions. Further, it would be interesting to compare the clinical outcomes of older PWP cared for in a CGA model vs. other settings to determine best practice for these patients.
Acknowledgments
The authors would like to acknowledge the contributions of Harindra Rajasekeran and Harrison Mah, our research assistants in data collection and administration of anonymous survey; and of Dr. Jeremy Theal and Dr. Mario Masellis for proofreading and technical editing of the manuscript.
Funding
Funding was provided by Exploration Fund at North York General Hospital, a competitive internal fund granted to hospital staff and physicians for innovative projects to improve patient care. Sponsor had no role in the design, methods, subject recruitment, data collection, analysis or preparation of paper.
Conflicts of Interest
Joyce Lee received honoria from Pfizer Pharmaceuticals for delivery of a CME presentation about Overactive Bladder in Parkinson’s Disease in 2017. Greta Mah – none to declare. Janis Miyasaki has received royalties from Up to Date for functional movement disorders chapter, and a grant from Patient Centered Outcomes Research Institute (NIH). Sumeet Kalia – none to declare.
Statement of Authorship
JWL: conception and design of study, application for funding, interpretation of data, preparation and approval of manuscript. GM: conception of study, collection of data, interpretation of data, preparation and approval of manuscript. JM: design of study; critical revision of manuscript for intellectual content; approval of final version. SK: data analysis and interpretation, preparation and approval of manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2020.253.









