Orthostatic myoclonus (OM) is a recently identified movement disorder that leads to a slowly progressive stance and gait impairment in the elderly.Reference Glass, Ahlskog and Matsumoto 1 It is described as jerkiness/tremulousness or unsteadiness while standing. The differential diagnosis includes mainly orthostatic tremor (OT) and other disorders characterized by similar gait and balance problems not diagnosed as another entity named as orthostatic hyperkinesia, at least some of which are functional movement disorders.Reference Leu-Semenescu, Roze and Vidailhet 2 , Reference van Gerpen 3 Electrophysiological evaluation enables differentiation of these conditions. It is probably an important cause of falls in the elderly and is frequently accompanied by gait initiation failure. OM may develop as a symptom of neurodegenerative disorders.
Herein we report the clinical and electrophysiological findings in seven patients with OM and assess its association with falls and neurodegenerative disorders.
We retrospectively assessed the medical records of patients who were referred to the Electromyography Laboratory of the Neurology Department at the Cerrahpaşa Medical Faculty with complaints of jerkiness/tremulousness or unsteadiness upon standing and irregular twitches on examination that was confirmed to be OM by electrophysiological analysis.
Demographic features (age, gender) and clinical features (accompanying neurological findings including cognitive assessment, systemic findings, clinical diagnosis) were retrieved from the medical records. In all patients, analysis of the surface electromyography of the lower limbs during sitting and standing, blink reflex (BR), auditory startle response (ASR), and long-latency reflexes (LLRs) were performed.
The first step in the electrophysiological examination was polymyography using cutaneous recording electrodes (Neuropack Sigma MEB-5504 k, Nihon Kohden Medical, Tokyo, Japan). Recording electrodes were placed on the bilateral or unilateral rectus femoris, anterior tibialis, and gastrocnemius muscles. In some cases, we also placed electrodes on the lumbar paraspinal, biceps brachii, triceps brachii, and wrist flexor or extensor muscles. The ground electrode was on the anterior side of the tibia. Frequency interval, sweep time, and sensitivity were adjusted as 20 Hz–5 kHz, 50 ms–200 ms/div, 200–1000 V/div, respectively. BR was obtained over the bilateral orbicularis oculi (Ooc) muscle by stimulating the supraorbital branch of the trigeminal nerve using a 0.2-ms single electrical stimulus with an intensity three times the sensory threshold. For ASR recordings, surface electrodes were placed over the unilateral masseter, Ooc, sternocleidomastoid, forearm flexor, and anterior tibial muscles while the ground electrode was placed over the sternum. The stimulus was a monophasic auditory stimulus (tone-burst: 1 kHz in frequency, 50-ms plateau time) delivered bilaterally through earphones as 8 bursts, with an intensity of 105 dB HL and at random intervals of 2–5 min. Single sweeps of 500 ms were recorded with filters set at 10 and 10,000 Hz. For LLRs, the recording electrodes were placed over the belly of the APB muscle with the ground electrode over the palm. The electrical stimulus (0.2 ms in duration) was randomly applied on the median nerve at the wrist with the stimulus intensity set between 15 and 20 mA during rest and while subjects were performing a slight (~10–25% of maximum) contraction. The recording was repeated 20 times. The bandpass filters were 2 to 2000 Hz.
Seven patients with OM were identified who were referred between June of 2010 and June of 2016. Their age range was 65–89 years. Some 4 patients were older than 80 years of age, and 4 patients were male. All patients except one had a neurological diagnosis (Table 1). Treatment with levodopa in patients with parkinsonism partially improved unsteadiness and decreased the frequency and severity of jerks. Treatment trials with piracetam in two patients and clonazepam in one provided no improvement.
Table 1 Demographical, clinical, and electrophysiological features of all patients
IPD=idiopathic Parkinson’s disease; LBD=Lewy body disease; LBP=lower-body parkinsonism; LD=levodopa; MY=myoclonus; NA=Not available; PSP=progressive supranuclear palsy; VaP=vascular parkinsonism.
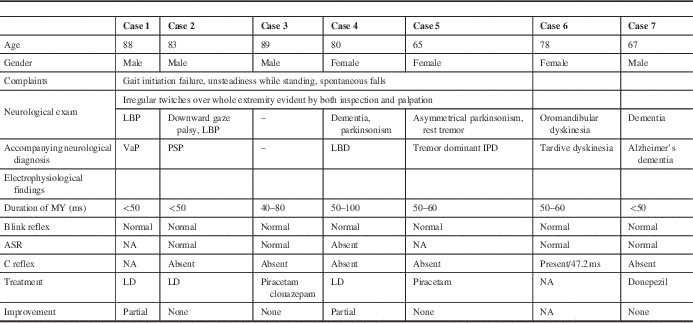
There were high-amplitude discharges (positive myoclonus) and interruptions of interference pattern (negative myoclonus). Positive myoclonus was short in duration in all patients, and it was recorded asymmetrically on the tibialis anterior muscle and gastrocnemius muscles solely while standing (Figures 1 and 2). The bursts were not stimulus-sensitive. One patient with accompanying oromandibular dyskinesia had the C reflex. Blink and auditory startle reflexes were normal in all patients except one, who had accompanying dementia and parkinsonism.
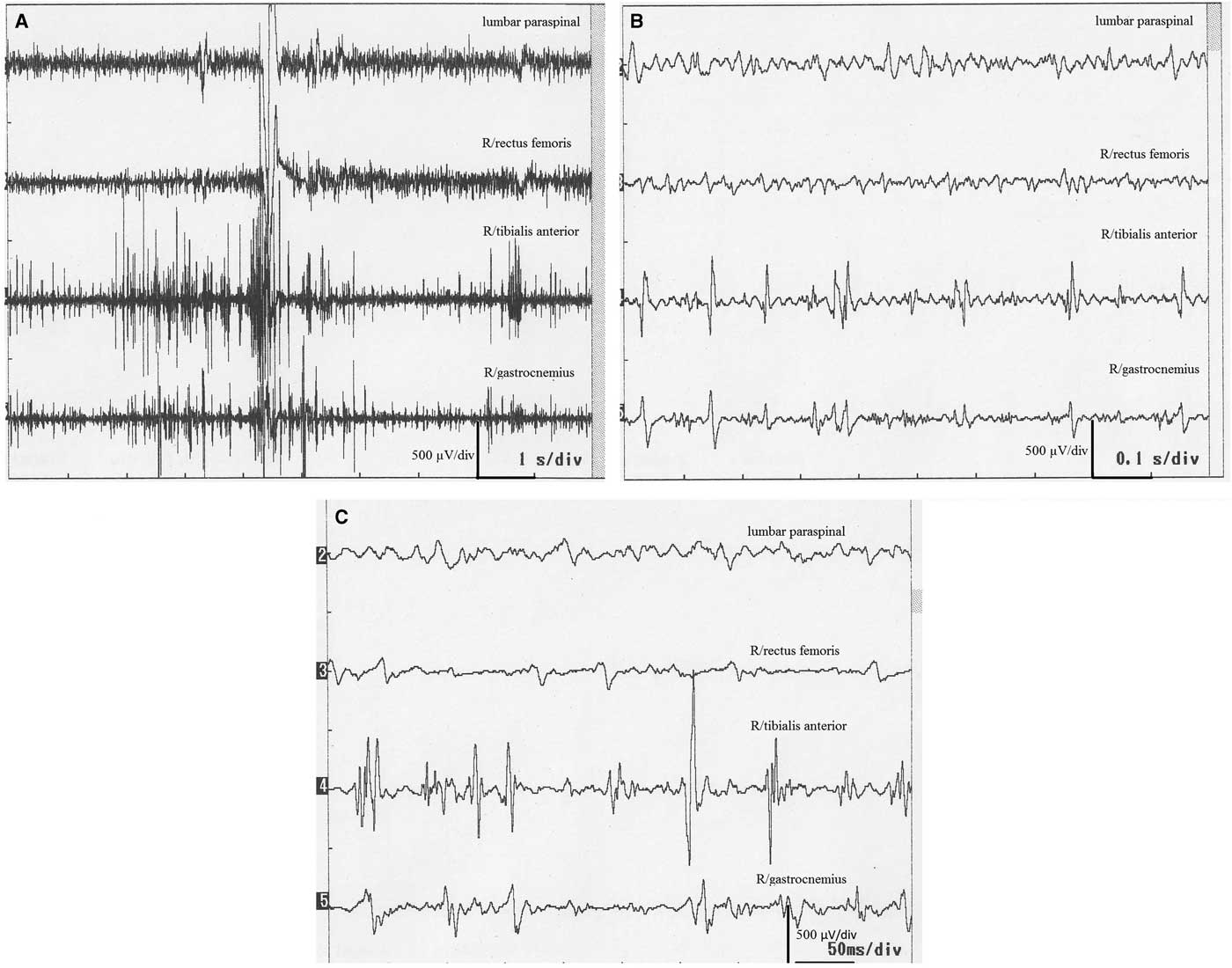
Figure 1 Surface electromyography recordings of an 83-year-old man (case 2). (A) Onset of myoclonic discharges on the tibialis anterior and gastrocnemius muscles followed by appearance of discharges on the rectus femoris when the patient started standing. (B,C) Appearance of myoclonic discharges during standing in more enlarged scales.
Figure 2 Surface electromyography recordings of a 78-year-old woman (case 6). (A,B) Myoclonic discharges during standing. Stars show positive myoclonus and arrows stand for interruption of the interference pattern (negative myoclonus).
The clinical features, treatment trials, and electrophysiological findings are presented in Table 1.
This small case series represents samples of unsteadiness due to OM. Patients with OM have complaints similar to those seen in OT: unsteadiness upon standing and quivering, trembling, or twitching of legs relieved by walking. Electrophysiological recordings are necessary for a differential diagnosis.Reference Glass, Ahlskog and Matsumoto 1
OM was first described in 2007, and there are only 5 reports since then covering approximately 100 patients with OM.Reference Gasca-Salas, Arcocha, Artieda and Pastor 4 , Reference Aldaajani, Chang, Kim, Mahant and Fung 5 One report, which illustrated 32 cases seen in an institution within only 30 months,Reference van Gerpen 3 suggested that the condition may be more prevalent than expected, even more prevalent than OT. The delay in recognition of OM by up to 11 years is probably related to a lack of awareness of the clinicians about the subject.
Unlike OT, involvement of the upper extremities is infrequent in OM, and OM is generally reported in conjunction with such neurodegenerative conditions as Parkinson’s disease, Parkinson-plus syndromes, dementias, or systemic diseases.Reference Glass, Ahlskog and Matsumoto 1 , Reference Leu-Semenescu, Roze and Vidailhet 2 , Reference Gerschlager, Münchau and Katzenschlager 6 Although there was one case of OM with Caspr2 antibodies involving the upper extremities,Reference Gövert, Witt and Erro 7 this diagnosis was challenged in a successive comment.Reference van Gerpen, Ahlskog and Chen 8 Glass and colleaguesReference Glass, Ahlskog and Matsumoto 1 classified some of their cases as idiopathic despite the presence of gait apraxia, abnormal autonomic testing, or radiological findings, including mild brain atrophy that may require follow-ups for possible development of degenerative diseases over time.
Myoclonus is classified according to origin of localization in the nervous system: cortical, corticosubcortical, brainstem reticular, segmental, and peripheral. Although the absence of somatosensory-evoked potentials is a limitation of our study, lack of the C reflex or stimulus sensitivity largely excludes cortical origin. The C reflex is long-latency reflex I, which is generally obtained on distal hand muscles after stimulating distal parts of peripheral nerves. It is generally present in cortical reflex myoclonus or corticobasal degeneration, reflecting hyperactivity in the cortex.Reference Cruccu and Deuschl 9 Presence of the C reflex in one patient probably may point to the simultaneous presence of another degenerative disorder or a possible cortical contribution in OM. The bursts in the brainstem reticular myoclonus follow an order, and each burst has longer duration.Reference Hallett, Chadwick, Adam and Marsden 10 Similarly, segmental myoclonus originating in the brainstem or spinal cord involves a specific pattern of muscles innervated by the segment of origin. Long and variable burst durations have been reported. Synchronous contractions of agonist and antagonist muscles occur sometimes in a semi-rhythmical fashion. When it is propriospinal myoclonus, there is an order of appearance of bursts over the involved muscles, and the conduction velocity is fast.Reference Chokroverty, Walters, Zimmerman and Picone 11 Negative myoclonus is yet to be reported in relation to propriospinal myoclonus. In our cases, bursts were very brief and had high amplitudes. The generation of bursts on the muscles did not follow any order. They mostly lacked coherence. Considering the coexistence of neurodegenerative disorders or microvascular leukoencephalopathy, as in the case of our illustrative patient, and electrophysiological findings such as very brief, high-amplitude, irregular, and incoherent bursts, a subcortical generator may be likely. Only one patient had the C reflex, and though she only had oromandibular dyskinesia at the time of investigation, the presence of the C reflex may be related to future development of another degenerative disorder. The C reflex was not recorded in other patients.
There is no specific medical treatment for OM. A trial of levodopa in selected patients with parkinsonism and use of gait-assistive devices may provide relief.Reference van Gerpen 3
Disclosures
Aysegul Gunduz, Melih Tutuncu, Burcu Zeydan, Hulya Apaydin, Gunes Kiziltan, Sibel Ertan, and Meral E. Kiziltan hereby declare that they have no conflicts of interest to disclose.
Statement of Authorship
MEK and AG were responsible for study concept and design. MT, BZ, GK, SE, and HA were responsible for case inclusion. MEK and AG were responsible for the electrophysiological recordings. AG was responsible for writing the first draft. MEK and AG were responsible for review and critique.







