INTRODUCTION
Ketamine is a phencyclidine and cyclohexylamine derivative, which was initially introduced as an anaesthetic agent in the 1970s but soon fell out of favour as newer anaesthetic agents with limited side-effect profiles were introduced. However, ketamine has a unique ability to create a dissociative state (a trance-like cataleptic state), which preserves airway reflexes and hemodynamic stability,Reference Thomas 1 while simultaneously providing potent analgesia, sedation, anxiolysis, and amnesia.Reference Green 2 These characteristics, coupled with its relatively fast onset and offset time of action and flexible route of administration (by mouth [PO], intravenous [IV], intramuscular [IM], by rectum [PR]),Reference Thomas 1 have made ketamine an attractive option for painful procedures requiring sedation and analgesia in the emergency department (ED).
There is ample evidence to support its use for procedural sedation and analgesia (PSA) in the pediatric population,Reference Green 2 but its use in adults has been slower to gain momentum due to reports of emergence reactions (anxiety, nightmares, hallucinations, delirium).Reference Chudnofsky, Weber and Stoyanoff 3 Many studies outlining the use, safety, and efficacy of ketamine for PSA in the adult ED populationReference Messenger, Murray and Dungey 4 - Reference Willman and Andolfatto 6 have led to the publication of a clinical practice guideline.Reference Green, Roback and Kennedy 7 Despite its growing popularity as a PSA agent, ketamine’s additional potential benefit exclusively as an analgesic or co-analgesic at sub-dissociative doses (<1 mg/kg IV or <2 mg/kg IMReference Willman and Andolfatto 6 , Reference Bell, Dahl, Moore and Kalso 8 , Reference Kochs, Scharein, Möllenberg, Bromm and Schulte am Esch 9 ) for acute pain management is a relatively new application within emergency medicine.
Pain management is an essential and challenging component of emergency medicine practice. There is a constant search for the ideal agent that will act quickly and provide almost instant analgesia with minimal side effects. Because low-dose ketamine (LDK) is a relatively new analgesic in the ED, its side effects and effectiveness as such an agent, including physician and patient satisfaction, have yet to be fully determined. The goal of this study is to systematically review the use of LDK as an analgesic or a co-analgesic for treatment of pain in the ED. We asked if, in adult patients requiring acute pain management in the ED (P), the use of LDK as an adjunct or alone (I), compared to using opioids (C), offered improved pain control, decreased the need for opioid analgesics, or decreased the occurrence of adverse events (O): PICO.
METHODS
This qualitative systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.Reference Moher, Liberati, Tetzlaff and Altman 10
Eligibility criteria of studies included
This qualitative systematic review studied the use of LDK in adult patients (>18 years old), requiring acute pain management for any condition in the ED. Eligible studies met the following criteria: LDK administered by any route (IV/IM/PO/SC), in any dose regimen (bolus or infusion), and compared to any opioid analgesics. LDK for analgesia is intended to be sub-dissociative and is typically defined as <1 mg/kg IV or <2 mg/kg IM.Reference Willman and Andolfatto 6 , Reference Bell, Dahl, Moore and Kalso 8 , Reference Kochs, Scharein, Möllenberg, Bromm and Schulte am Esch 9 The outcomes of interest were 1) analgesic effect of ketamine; 2) requirement of rescue analgesia; and 3) neuropsychiatric adverse events in patients who received ketamine. We excluded pediatric patients (<18 years of age), use of ketamine for procedural sedation, use of ketamine in a non-ED setting (inpatient, prehospital, emergency medical services), and use of ketamine for uses other than analgesia (e.g., perioperative, psychiatric, rapid sequence intubation, chronic pain).
Information sources and search strategy
We designed an electronic search strategy with the assistance of an information specialist using a combination of Medical Subject Headings (MeSH) and text words for the concepts identified in our PICO question. In February 2015, we searched MEDLINE (Ovid), EMBASE, AMED, CINAHL, and PubMed (all available records for each database since their creation up to and including February 2015). The search was limited to human studies, randomized controlled trials (RCTs), observational studies, and English language without further limitations of publication year.
We searched the National Institute of Health Trial Registry (clinicaltrials.gov), Cochrane Controlled Trial Registry, and the Cochrane database of systematic reviews. We also hand-searched abstracts from 2012 to 2014 for the Canadian Association of Emergency Physicians Conference, American College of Emergency Physicians Scientific Assembly, Society of Academic Emergency Medicine Annual Meeting, Canadian Anesthesiologists’ Society Annual Meeting, and American Society of Anesthesiologists Annual Meeting. We reviewed the bibliographies of the included full-text articles for any citations that may have been missed by the electronic search strategy. We also contacted main authors to identify unpublished reports.
Study selection
Titles and abstracts from the electronic database search results were imported into a bibliographical database library using EndNote version X7 (Thomson Scientific, Carlsbad, California). Duplicates identified by the EndNote software were automatically removed.
Two reviewers (GG, EC) independently assessed the titles and abstracts using the inclusion criteria previously described. For the initial selection, all articles with any disagreement and those that could not be decided based on title and abstract alone were included, and full texts of all of the selected articles were obtained. We calculated inter-rater agreement using kappa statistics after the initial review. The second and final selection of articles was completed by one reviewer (GG) based on a set of standardized and piloted criteria after reviewing full-text articles from the initial selection. Equivocal decisions on inclusion were reached by consensus among all investigators.
Data collection process and data items
Two data abstractors (GG, EC) created and piloted a data abstraction tool/form to ensure that this tool included all of the elements required before proceeding with standardized data extraction. This was done in accordance with the systematic-review methodology described in the PRISMA Statement.Reference Moher, Liberati, Tetzlaff and Altman 10
The following data were collected from studies deemed eligible after the second review: year, country and language of publication, study design, population characteristics, setting, LDK dose/route/need for re-dosing, LDK use for analgesia or procedural sedation, adjunct dose/route/need for re-dosing (e.g., opioids+/-benzodiazepines), comparator analgesic dose/route/need for re-dosing (e.g., opioids), pain score and scale used (Numerical Rating Scale [NRS]/Visual Analogue Scale [VAS]), type of pain treated, degree of pain relief, and adverse outcomes. Outcome measures recorded were analgesic effect of LDK in terms of patient-reported pain scores, “rescue” analgesia (i.e., the need for adjunctive pain management, primarily opioids, in this systematic review, to meet adequate qpain control in patients who receive only LDK) required in LDK used in analgesia, and incidence of neuropsychiatric adverse events observed in patients who received LDK. The data collection form was piloted by two reviewers.
Synthesis of results
We used the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) software to estimate the strength of recommendation of each study based on the quality of evidence and bias associated with studies included in the final selection. Meta-analysis was not possible due to a large variability in reported outcome measures, dose/interval/routes of LDK used, comparator analgesic (type and dose), and indications for LDK. Hence, we performed a qualitative analysis of all of the RCTs and observational studies included in this systematic review.
Quality assessment of studies
The quality of evidence of the selected RCTs and observational studies was assessed using the Cochrane Collaboration’s Tool and the GRADE software. The risk of bias in individual RCTs was assessed using the Cochrane Collaboration’s Tool by analysing the outcomes of interest in six main domains: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. The risk of bias in individual non-RCTs was also assessed using the Cochrane Collaboration’s Tool by analysing the outcomes of interest in four main domains: appropriate development of eligibility criteria, exposure/outcome measurement, confounding bias, and completeness of follow-up. These domains were used to summarize the bias in each study as low, unclear, or high.Reference Higgins, Altman, Gøtzsche and Jüni 11 This bias assessment was then extrapolated as one of the criteria for quality of evidence assessment across studies described in the following paragraphs.
The GRADE software was used to create an evidence profile that assessed and summarized the overall quality of evidence across all studies for each of the following criteria: study design, risk of bias in individual studies, inconsistency, indirectness of evidence, imprecision, and publication bias. Based on the assessment of each criterion for every study and outcome, the GRADE software generated an estimation of the quality of evidence across the studies for each outcome as “high,” “moderate,” “low,” and “very low.”
RESULTS
Study selection and characteristics
The search strategy yielded 1,413 potential articles: 1,408 from the electronic search and 5 from grey literature. After removing duplicates, there were 1,396 articles to review. The initial review of titles and abstracts excluded 1,352 articles and yielded 44 eligible articles (kappa=0.70; 95% CI 0.53-0.78). Thirty-eight articles were excluded for reasons indicated in the study flow diagram (Figure 1). Two eligible articles-in-press were provided directly by a main author after the initial review. Ultimately, six RCTs and two observational studies were included for the final qualitative analysis for a total of 609 patients.
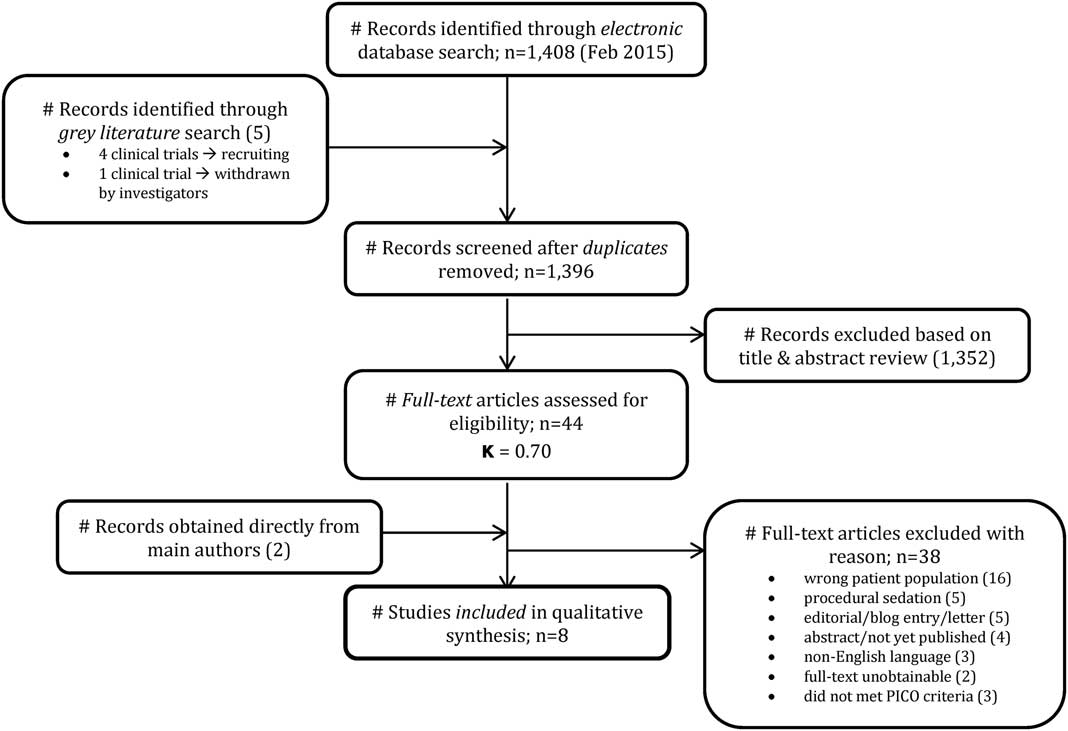
Figure 1 Study flow diagram.
General characteristics of all included studies are presented in Table 1. All articles were published in English between 1996 and 2015.Reference Ahmadi, Isfahani and Feizi 12 - Reference Yeaman, Meek and Egerton-Warburton 22 Six RCTsReference Ahmadi, Isfahani and Feizi 12 - Reference Motov, Rockoff and Cohen 17 included in the analysis had sample sizes from 40 to 236 and used LDK boluses of 0.15-0.30 mg/kg IV. One RCT used an infusion of 0.1 mg/kg/h IV.Reference Gurnani, Sharma and Rautela 15 They all used morphine boluses 0.1 mg/kg IV as a comparator. The two observational studiesReference Ahern, Herring and Stone 18 - Reference Yeaman, Meek and Egerton-Warburton 22 included in the analysis had sample sizes from 30 to 35. They both used LDK boluses of 0.1-0.7 mg/kg IV and a variety of opioid comparators.Reference Ahern, Herring and Stone 18 - Reference Yeaman, Meek and Egerton-Warburton 22
Table 1 Characteristics of the 8 included studies

RCT=randomized controlled trial.
Risk of bias within studies
A summary of the risk of bias assessment within individual studies is summarized in Table 2 based on the domains described in the Methods section. Overall, the RCTs included in the final analysis were rated to have a low risk of bias. However, we rated the RCTs to have an unclear amount of bias (under “other biases”) because their main outcomes (i.e., pain scores) were patient-reported. The non-RCTs had an overall low risk of bias, except for confounding bias because not all plausible prognostic factors in these observational studies were accurately measured.
Table 2 Risk of bias assessment

ED=emergency department; RCT=randomized controlled trial.
Results of individual studies
Details of population, LDK dose/route, comparator analgesic dose/route, and main findings of all included studies are summarized in Table 3. The main results for each outcome are discussed in the following paragraphs.
Table 3 Drug administration and main findings from the 8 included studies

ED=emergency department; IM=intramuscular; IN=intranasal; IV=intravenous; LDK=low-dose ketamine; MSK=musculoskeletal; RCT=randomized controlled trial; SC=subcutaneous.
a Initial bolus of 0.25 mg/kg IV given prior to SC infusion.
b Thirty out of 35 patients received IV; the rest received IM.
Analgesic effect of LDK based on patient-reported pain scores
All of the RCTs compared the analgesic effects of LDK (0.15-0.30 mg/kg IV) to those of morphine (0.1 mg/kg IV), and they all concluded that IV boluses of LDK were safe and effective, either on their own or in conjunction with IV morphine for a variety of acute pain conditions seen in the ED.Reference Ahmadi, Isfahani and Feizi 12 - Reference Motov, Rockoff and Cohen 17 They also measured pain relief in the form of patient-reported pain scores (NRS or VAS) at 30 minutes post-LDK administration, as presented in Table 4. None of the studies demonstrated a significant difference in reported pain scores at T30Reference Ahmadi, Isfahani and Feizi 12 - Reference Galinski, Dolveck and Combes 14 , Reference Motov, Rockoff and Cohen 17 or maximum change in pain scoresReference Miller, Schauer and Ganem 16 between the LDK and morphine groups, except for one RCT where the pain scores were consistently lower (i.e., consistently less pain) in the LDK group over a 24-hour period.Reference Gurnani, Sharma and Rautela 15 One RCT concluded that LDK had an opioid-sparing effect.Reference Galinski, Dolveck and Combes 14
Table 4 Analgesic effect of ketamine in terms of patient-reported pain scores

CI=confidence interval; ED=emergency department; LDK=low-dose ketamine; N/A=not applicable; No.=number; NRS=Numerical Rating Scale; RCT=randomized controlled trial; SPID=summed pain intensity difference; T30=30 minutes; VAS=Visual Analogue Scale.
Although the observational studies did not directly compare the analgesic effectiveness of LDK to opioids (used as adjuncts to LDK in some studies),Reference Ahern, Herring and Stone 18 , Reference Ahern, Herring, Miller and Frazee 19 they concluded that LDK is a reasonable and effective option in acute pain management in the ED.Reference Ahern, Herring and Stone 18 - Reference Yeaman, Meek and Egerton-Warburton 22 However, as indicated in Table 4, the quality of these papers was graded as “very low.”
Although none of the studies observed a significant change in pain scores in patients receiving LDK (as an adjunct) at T30, they did suggest that LDK had a morphine-sparing effectReference Beaudoin, Lin and Guan 13 , Reference Galinski, Dolveck and Combes 14 with significant analgesic effect at 5 minutes post-analgesic administrationReference Miller, Schauer and Ganem 16 and 2-hours post-analgesic administrationReference Beaudoin, Lin and Guan 13 compared to morphine’s analgesic effect at the same time intervals. There is a moderate level of evidence in support of using LDK as an adjunct to opioids for acute pain management in the ED.
Rescue analgesia required with LDK
Table 5 summarizes the mean rescue analgesia dose/route and the number of patients in each group who received rescue analgesia.
Table 5 Requirement of rescue analgesia in patients treated with LDK
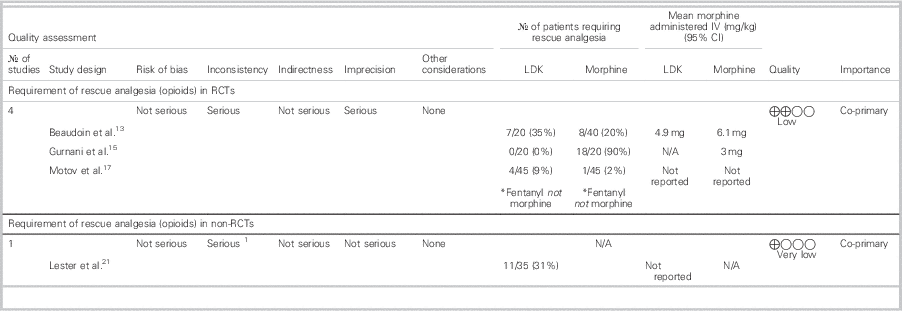
CI=confidence interval; IV=intravenous; LDK=low-dose ketamine; N/A=not applicable; No.=number; RCT=randomized controlled trial.
In the RCT by Gurnani et al., supplemental morphine was not required at all by any patients in the LDK group.Reference Gurnani, Sharma and Rautela 15 Beaudoin et al. and Motov et al. reported the need for rescue analgesia as a secondary outcome.Reference Beaudoin, Lin and Guan 13 , Reference Motov, Rockoff and Cohen 17 Beaudoin et al. used morphine (0.05 to 0.1 mg/kg IV q1h) 30 minutes after initial doses were administered in each treatment arm (morphine+placebo v. morphine+0.15 mg/kg ketamine v. morphine+0.30 mg/kg ketamine) and did not observe a significant difference in the requirement of rescue analgesia between all three groups. Furthermore, due to the small number of patients who needed rescue analgesia, there was inadequate power to detect a difference in receipt of rescue analgesia between LDK versus morphine groups.Reference Beaudoin, Lin and Guan 13
Motov et al. administered fentanyl (1 ug/kg), if requested by patients, at 30 or 60 minutes after morphine or LDK was administered to their respective randomized group. They did not observe a statistically significant difference between the morphine and LDK groups for the use of rescue analgesia at 30 minutes (% difference=7.0%; 95% CI -3.0 % to 16.0 %) or 60 minutes (% difference= -5.0%; 95% CI -18.0 % to 9.0 %). However, they did note that, at 120 minutes, the ketamine group required significantly more rescue fentanyl (% difference=17.0%; 95% CI 1.0 % to 34.0 %) than the morphine group.Reference Motov, Rockoff and Cohen 17
One health record reviewReference Lester, Braude and Niles 21 reported that a significant portion of their study patients receiving only LDK required rescue analgesia.
The low quality of all of the data for rescue analgesia is attributable to the heterogeneity in reporting the use of rescue analgesia. Ultimately, if LDK is used for acute pain management in the ED, opioids may be necessary for rescue/additional analgesia.
Incidence of neuropsychological adverse events in patients who received LDK
Because the major barrier for clinicians to use LDK over opioids is the possibility of emergence reactions, we thought it was more important to focus on adverse events specific to these emergence reactions. It is worth noting that there were no significant differences in respiratory depression, which is a commonly feared side effect of opioid use, between the LDK and opioid groups in all of the RCTs.Reference Ahmadi, Isfahani and Feizi 12 - Reference Galinski, Dolveck and Combes 14 , Reference Miller, Schauer and Ganem 16 , Reference Motov, Rockoff and Cohen 17
A wide range of definitions of “neuropsychological adverse events” across all studies precluded a meta-analysis, thereby generating a “generalized” adverse event rate. The observed rate of adverse events varied from 0% to 75% of LDK cases across all eight studies. The following symptoms were analysed in this review: for the RCTs – agitation, hallucinations, dysphoria, confusion; and for the observational studies – agitation, hallucinations, dysphoria, confusion, dizziness (Table 6).
Table 6 Neuropsychological adverse events in patients treated with LDK for analgesia

CI=confidence interval; LDK=low-dose ketamine; N/A=not applicable; No.=number.
All of the RCTs except for one (in which midazolam was administered in the LDK group pre-emptively to avoid emergence reactions)Reference Ahmadi, Isfahani and Feizi 12 reported that a number of patients from the LDK group experienced some degree of dysphoria, hallucinations, agitation, and/or confusion. Galinski et al. noted neuropsychological adverse events in the LDK group but also noted that, overall, patient satisfaction was not significantly different between the LDK and morphine group.Reference Galinski, Dolveck and Combes 14 One RCT with a small sample size noted that, although the LDK group experienced dysphoria, lightheadedness, hallucinations, dizziness, and/or drowsiness, midazolam was not used (part of their protocol), because there were no incidences of dissociation or emergence reactions.Reference Miller, Schauer and Ganem 16 In one RCT, there was initially a statistical difference between groups that was not sustained at 15 and 30 minutes post-injection.Reference Motov, Rockoff and Cohen 17 Another RCT reported that only 2 out of 40 patients experienced “dreams” after the initial bolus dose of ketamine, which was statistically insignificant.Reference Gurnani, Sharma and Rautela 15
A significant portion of patients in the prospective cohort study by Ahern et al. experienced neuropsychological adverse events that were “self-limited psychomimetic side effects.”Reference Ahern, Herring and Stone 18 In the other retrospective case series, 1 patient out of 35 reported having “brief mild dysphoria”Reference Lester, Braude and Niles 21 but no other dangerous adverse events.
There is a moderate level of evidence reporting low incidence neuropsychological side effects, therefore supporting the use of LDK for analgesia in the ED.
DISCUSSION
Summary of evidence
The objective of this study was to systematically review the use of LDK (0.15-0.30 mg/kg IV) as an analgesic or co-analgesic for the acute pain management of adult patients in the ED. Our findings are summarized as follows: 1) LDK has an inconsistent but potentially rapid onset of analgesic effect. Although short-lived, this quick analgesic effect often reduces the required doses of opioids for adjunct analgesia; 2) patients receiving only LDK for pain management may require administration of rescue analgesia in the form of opioids to adequately control their pain; and 3) neuropsychological adverse effects of LDK are often self-limited, not life-threatening, with no significant emergence reactions reported out of 609 patients in the studies included in this review. Our findings suggest that LDK is a relatively safe and opioid-sparing alternative for acute pain management in adult patients in the ED.
A similar systematic review and meta-analysis by Lee et al.Reference Lee and Lee 23 that also focused solely on the use of LDK for acute pain has three major differences compared to this review. Firstly, Lee et al.Reference Lee and Lee 23 included two RCTs by Messenger et al. and Jennings et al. that were excluded in this review based on our clearly defined PICO. The RCT by Messenger et al.Reference Messenger, Murray and Dungey 4 included patients over the age of 16 years, whereas our inclusion criteria was for a population over age 18 years. The RCT by Jennings et al.Reference Jennings, Cameron and Bernard 24 was excluded based on setting; it was conducted out-of-hospital and not in an ED. Secondly, given the grossly heterogeneous data, we believed that a meta-analysis was not justifiable and that a narrative review was the only methodologically sound way of reporting our findings. Thirdly, Lee et al.Reference Lee and Lee 23 used two reviewers for study selection and data extraction. Two reviewers were involved in our study selection process, and the data were extracted by one reviewer only. All study results were obtained directly from the reviewed articles and were not subject to interpretation, thereby mitigating the need for the data to be extracted separately by two reviewers.
A few limitations of the review by Lee et al.Reference Lee and Lee 23 are also worth noting: 1) They failed to define a clear PICO, hence their reason for the inclusion of the RCT by Jennings et al.,Reference Jennings, Cameron and Bernard 24 but exclusion of other out-of-hospital studies is unclear; 2) our methodology is more robust – explicit inclusion/exclusion criteria and thorough search methods; and 3) we used the GRADE software to assess quality of evidence and risk of bias in each study.
In 2015, a review of four RCTs by Sin et al.Reference Sin, Ternas and Motov 25 (three adult and one pediatric; two of the four RCTs are included in our review,Reference Galinski, Dolveck and Combes 14 , Reference Gurnani, Sharma and Rautela 15 whereas the other two were ineligible for our review) showed no detectable differences in pain scores between the analgesic effect of LDK and opioids. They concluded that LDK could produce satisfactory pain control and could be opioid-sparing. Furthermore, adverse events resulting from LDK use were limited and did not require any intervention.Reference Sin, Ternas and Motov 25 Emergence phenomenon was reported in one patient in the pediatric RCT.Reference Kennedy, Porter and Miller 26
Another RCT by Messenger et al. in 2008, which was excluded in our review due to its inclusion of pediatric patients, concluded that 0.3 mg/kg IV “sub-dissociative dose” of ketamine is safer than fentanyl plus propofol in PSA. Also, no emergence reactions were observed, which was attributed to the small sample size and LDK by the original study authors.Reference Messenger, Murray and Dungey 4
LIMITATIONS
This systematic review has a few limitations that we wish to acknowledge. Firstly, there was a single reviewer for the final inclusion criteria. However, this limitation is mitigated by stringent predetermined inclusion/exclusion criteria. Secondly, the strength of recommendation regarding the adverse effects of LDK was limited by the small number of papers (and small sample sizes within each study) reporting such an outcome. Thirdly, only neuropsychological adverse events were analysed and reported in this review, so it is important to note that other side effects, such as respiratory depression and gastrointestinal symptoms, may still be seen with LDK.
CONCLUSIONS
Our findings, despite being derived from moderate to very low levels of evidence, suggest that LDK could be considered as an effective and opioid-sparing adjunct with some risk for neurophsychological side effects in acute pain management in the adult patients in the ED.
There is an opportunity for a well-conducted RCT with a larger sample size and rigorous methodology to further elucidate the true extent of LDK’s neuropsychological side effects and analgesic profile for acute pain in the ED.
Acknowledgements
We would like to acknowledge the contribution of Ms. Alexandra Davis, Information Specialist, for her assistance in devising our electronic search strategy and Ms. Angela Marcantonio for her assistance in the submission process.
Competing interests: None declared.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cem.2017.48











