Introduction
The Dakota skipper, Hesperia dacotae (Skinner, 1911) (Lepidoptera: Hesperiidae) is an at-risk prairie-obligate Lepidoptera species that inhabitants native mesic mixed-grass prairie (Committee on the Status of Endangered Wildlife in Canada 2014). Klassen et al. (Reference Klassen, Westwood, Preston and McKillop1989) and Layberry et al. (Reference Layberry, Hall and Lafontaine1998) describe the male Dakota skipper as a yellowish-orange to orange butterfly containing a black brand on its forewing with occasional dull spots on its hindwing. The female Dakota skipper is greyish brown to brown with reduced pale spots on both the forewing and the hindwing (Klassen et al. Reference Klassen, Westwood, Preston and McKillop1989; Layberry et al. Reference Layberry, Hall and Lafontaine1998). The species spends the majority of its life as a larva, occupying soil level in the winter months and above soil surface in the summer months, where it feeds and constructs a shelter made of native prairie host plants. The adult Dakota skipper is dependent on diverse prairie vegetation for nectar resources and mating perches (Dana Reference Dana1991). These life stage characteristics limit the Dakota skipper to high-quality native prairie (Webster Reference Webster2007; Westwood Reference Westwood2010; Committee on the Status of Endangered Wildlife in Canada 2014) within the moist-mixed and mixed-grass ecoregion (Acton et al. Reference Acton, Padbury and Stushnoff1998). The Dakota skipper is declining in both distribution and abundance (Layberry et al. Reference Layberry, Hall and Lafontaine1998; Committee on the Status of Endangered Wildlife in Canada 2014), presumably due to declines in suitable habitat. Currently, a lack of knowledge exists about the environmental associations of the Dakota skipper in southeastern Saskatchewan. The Saskatchewan Dakota skipper population was confirmed in 2001 and limited survey data are available (Hooper Reference Hooper2003). Saskatchewan presents a unique opportunity for Dakota skipper conservation as it contains the largest portion of remaining mesic mixed-grass prairie within the species distribution (Bailey et al. Reference Bailey, McCartney and Schellenberg2010), where the Dakota skipper population inhabits the extreme northwestern extent of its known distribution (Committee on the Status of Endangered Wildlife in Canada 2014), and additional unidentified habitat and populations may exist.
The objective of this research is to characterise the environmental associations of the Dakota skipper in southeastern Saskatchewan. The Committee on the Status of Endangered Wildlife in Canada (2014) and the United States Fish and Wildlife Service (2015) state that critical habitat is an area that contains features essential to the survival of a species. Features of this critical habitat can include the environmental associations of a species; therefore, environmental associations of the Dakota skipper are features of the environment needed for the species to persist and inhabit an area. Defining the environmental associations of the Dakota skipper in southeastern Saskatchewan may help identify suitable habitat and identification of new populations, contributing to the overall understanding and conservation of this species.
Material and methods
Study region
The study region was selected from the known distribution of existing Dakota skipper populations in the Souris River Valley of the southeastern Saskatchewan mesic mixed-grass ecoregion (Environment Canada 2007; Committee on the Status of Endangered Wildlife in Canada 2014). This ecoregion is located in a semiarid climate, with a climate normal mean annual precipitation of 433 mm (Environment Canada 2017). Elevations range between 520 and 580 m within the Souris River Valley. The valley is dominated by dark brown soils developed in glacial till parent material. Agriculture makes up 80% of the land use of the mixed-grass ecoregion, while the remainder consists of natural vegetation cover, wetlands, and industrial activity such as oil, gravel, gas, and coal (Acton et al. Reference Acton, Padbury and Stushnoff1998).
Study site selection
Survey sites were located within a 3.2 km buffer of the Souris River channel (Fig. 1). Through examination of Google Earth (https://earth.google.com/web) aerial imagery from 2016, land cover was initially characterised for potential survey sites identified within this buffer. Potential survey sites were quarter sections (65 ha) that contained approximately 20% or greater of native, tame, or hay land covers, as other land cover types (e.g., annual cropland) were not expected to support the Dakota skipper (Westwood Reference Westwood2010). A random number generator was used to select a subset of survey sites from the list of potential sites. Landowners were identified and contacted in order to obtain permission for land access. If land access was denied, the next site on the list was selected.
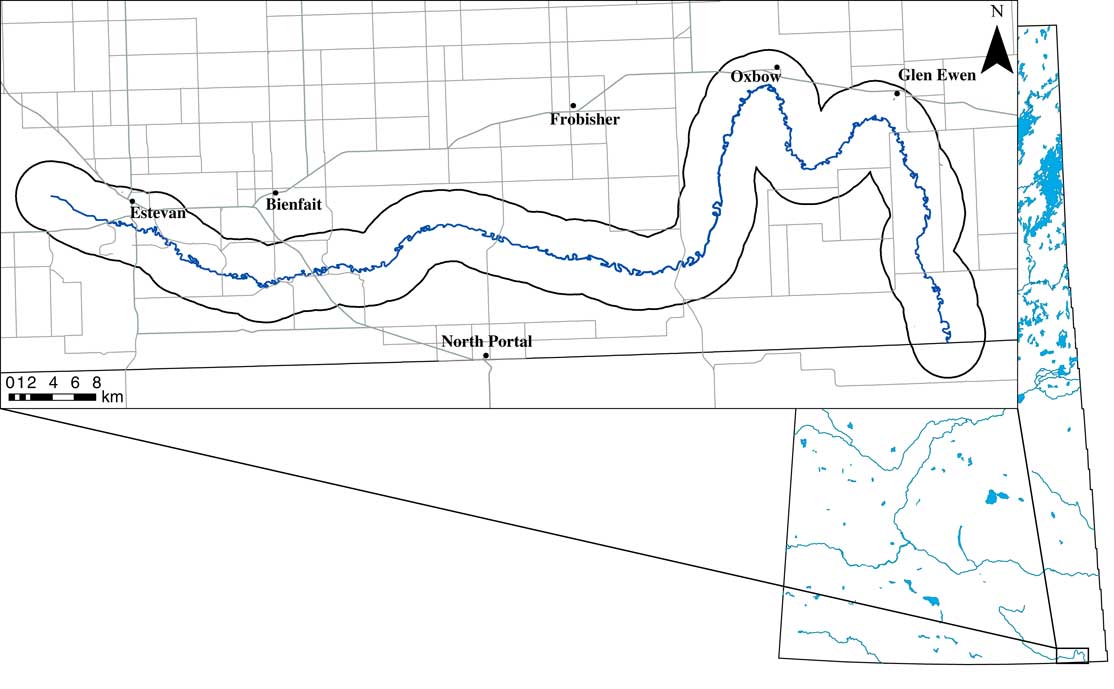
Fig. 1 The study area located in southeastern Saskatchewan within the Souris River Valley (right). An insert of the Souris River shows the 3.2 km (2 miles) study area buffer around the Souris River channel (left). A total of 46 sites were surveyed in this area during the 2015 and 2016 field season.
Landscape and land cover survey
Once on site, land use and land cover were validated for accuracy and study-appropriateness by visual assessment and included in surveys if they contained appropriate land cover of native grassland, tame, or hay (Westwood Reference Westwood2010). Land cover was classified as native when the majority of vegetative species were native mesic mixed-grass prairie species; invaded native when the majority of the species were introduced or tame species, with no evidence of tilling or soil disturbance; tame when the majority of the species were introduced or tame species and the soil contained evidence of disturbance or tilling (Saskatchewan Conservation Data Centre 2017). Land cover was classified as hay on tame land cover that is cut annually or semi-annually. Elevation was obtained from the Natural Resources Canada spatial climate model (McKenney et al. Reference McKenney, Hutchinson, Papadopol, Lawrence, Pedlar and Campbell2011). Slope was determined using a compass clinometer, taken at the start of each transect and measured to the height of the steepest slope within the survey transect. Heat load values were calculated for the centre of each site based on McCune and Keon (Reference McCune and Keon2002). Landscape variables used in the analysis include elevation, degree of slope, heat load, per cent introduced and native plant species, and total species richness.
Vegetation survey
Survey sites were selected based on the representative plant community observed within the targeted survey quarter section during field observations. Once a site was selected, a 250-m transect was staked out where 1 m2 plant-survey quadrats were placed at 50-m intervals on the transect, for a total of six 1-m2 quadrats (Fig. 2) (Rigney Reference Rigney2013). Within each survey quadrat all plants were identified to species and foliar per cent cover visually estimated (Saskatchewan Conservation Data Centre 2017). Plant species that could not be identified in the field were collected for later verification. Plant data were averaged to the site level and total species richness was determined for each survey site. Plant species list is provided in Supplemental Table 1, and plant species cover is provided in Supplemental Table 2.
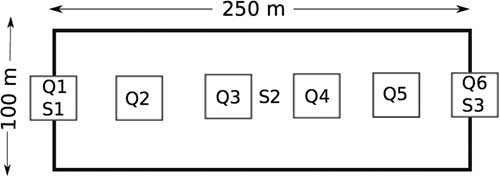
Fig. 2 Example of a typical survey site with the Hesperiidae survey area (100×250 m) including the vegetation quadrats (Q) and soil samples (S) running down the centre. All sites target a slope; starting at the toe slope (Q1; S1), mid-slope (S2), and upper slope (Q6; S3) when possible (not to scale).
Soil survey
Soil surveys were conducted along the vegetation transect (Fig. 2) with a total of three soil profiles classified and sampled at each survey site. Soil sample locations were selected based on landform changes, targeting an upper slope, mid-slope, and toe slope to fully capture the site-level variation. Soil profiles were classified on site through soil augering and soil pits; an auger sample was taken from each profile at an interval of 0–15 cm. Soils were described and classified according to the Canadian System of Soil Classification (Soil Classification Working Group 1998). Ground litter measurements were taken with a measuring tape at each soil sample site and bulk density samples were taken using a bulk density hand punch for the interval of 0–15 cm.
In 2015, soils were air-dried and ground to pass through a 2-mm sieve, then analysed for pH, electrical conductivity, sodium, calcium, magnesium, potassium, phosphorous, organic carbon (C), and inorganic C. Initial statistical analysis of 2015 soils determined these variables to be unlikely environmental associations of the Dakota skipper. Soil samples for 2015 and 2016 survey sites were analysed for gravimetric water content of field-moist and air-dried soils, particle analysis, nitrogen, and total C. All soil variables were averaged at the site level; variables used in the analysis include bulk density, gravimetric field-moist and air-dried soil moisture, per cent sand, silt, and clay content, organic C,
![]() ${\rm NH}_{4}^{{\plus}} $
and
${\rm NH}_{4}^{{\plus}} $
and
![]() ${\rm NH}_{3}^{{\minus}} $
, A horizon depth, and litter depth. All soil methods, analysis, and citations are provided in Supplemental Table 4.
${\rm NH}_{3}^{{\minus}} $
, A horizon depth, and litter depth. All soil methods, analysis, and citations are provided in Supplemental Table 4.
Hesperiidae butterfly surveys
Hesperiidae butterfly surveys were conducted among the vegetation and soil transect at each site (Fig. 2). Surveys were conducted between 29 June 2015 to 29 July 2015 and 3 July 2016 to 20 July 2016. Survey methods followed those of Westwood (Reference Westwood2010). In brief, an area of 250×100 m was staked out the night or morning before the survey; care was taken not to disturb the survey area. Surveys were conducted between the hours of 9:00 and 18:00 when temperatures had reached or exceeded 20 °C in sunny or cloudy weather with a wind speed less than 20 km/hour; the optimal conditions for adult Hesperiidae to be in flight. Two observers walking side by side observed an area of ~5 m ahead and 5 m to each side. Butterfly nets were used to capture adult specimens, which were released immediately after identification and photographic records taken. Surveys were carried out for a total of 30 minutes for each survey site. Survey time was limited to search time and excluded time spent pursuing and identifying a specimen. In 2015, two surveys were carried out at each site a minimum of one week apart; in 2016 to maximise survey coverage, only one survey was carried out at a site if a Dakota skipper observation was made on the first survey. Identification of a single Dakota skipper butterfly confirmed the presence of this species at that location, and thus the site was scored as a positive site. When no Dakota skipper butterflies were observed following the survey protocol, the species is assumed to be absent and the site is considered negative. Surveys targeted the Dakota skipper; however, similar species of Hesperiidae, including Peck’s skipper, Polites peckius (Kirby, 1837), long dash skipper, Polites mystic (Edwards, 1863), European skipper, Themelicus lineola (Ochsenheimer 1808), and tawny-edged skipper, Polites themistocles (Latreille, 1824) were also captured and recorded (Supplemental Fig. 1). All sites are analysed based on species detected presence. Hesperiidae butterfly observations are provided in Supplemental Table 7.
Microclimate survey
Microclimate monitoring was conducted during the 2016 growing season at the 2015 sites to determine if there were ground-level temperature differences between Dakota skipper positive and negative sites. One to three Logtag temperature recorders were placed on the soil surface of each 2015 site on 30 April 2016 and 1 May 2016 and recovered on 19 September 2016 and 20 September 2016. During this period, data loggers recorded air temperature (°C) at half-hour intervals. All negative Dakota skipper sites had one temperature logger that was placed in the middle of the vegetation transect. Positive Dakota skipper sites had two to three temperature loggers placed at even intervals along the transect to ensure successful collection of microclimate data in the limited positive sites, in the event that a data logger malfunctioned or could not be recovered; as there were few within-site differences temperatures were later averaged to the site level. Variables analysed include maximum daily temperature (°C), minimum daily temperature, and average daily temperature. All temperature logger data are provided in Supplemental Table 8.
Climate
Climate normal data were obtained from the Natural Resources Canada spatial model of growing season variables for Canada as described in McKenney et al. (Reference McKenney, Hutchinson, Papadopol, Lawrence, Pedlar and Campbell2011); 10 km gridded data were obtained for the study region for the climate normal period of 1981–2010. A value was assigned to each site to determine if there were climate normal differences between Dakota skipper positive and negative sites. Variables include annual mean temperature (°C); mean diurnal range; isothermality; temperature seasonality; maximum temperature during the warm period; minimum temperature during the cold period; temperature range; average temperature during the wettest, driest, warmest, and coldest quarter; annual precipitation (mm); precipitation during the wettest and driest period; seasonal precipitation; precipitation during the wettest, driest, warmest, and coldest quarter; Julian days since the start and end of the growing season; total amount of growing season days; average precipitation; annual minimum and maximum temperature; monthly minimum and maximum temperatures; and monthly precipitation.
Statistical analysis
All variables were transformed to a 0-centred standard normal deviate, averaged, and analysed at the site level. Differences in vegetative communities between Dakota skipper positive and negative sites were explored using nonmetric multidimensional scaling through R library vegan (R Core Team 2015; Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre and McGlinn2016); a permanova was used to test for significant differences in community composition. Associations of individual plant species with Dakota skipper presence were assessed through an indicator species analysis through R library labdsv (R Core Team 2015; Roberts Reference Roberts2016). Climate normal, soil, and landscape variables for Dakota skipper positive and negative sites were explored using nonmetric multidimensional scaling through R library vegan (R Core Team 2015; Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre and McGlinn2016); a permanova was used to test for significant differences in Dakota skipper positive and negative sites. A generalised linear model with a binomial distribution was fit for using function glm on each soil and landscape variable to determine whether individual soil and landscape variables significantly predicted Dakota skipper presence. Site-level heterogeneity in soil and landscape variables were examined through an analysis of coefficients of variation. Coefficients of variation were calculated using the R library goeveg (R Core Team 2015; Goral and Schellenberg Reference Goral and Schellenberg2017). Comparison of microclimate variables between Dakota skipper positive and negative sites were explored using generalised linear mixed models fit using function glmer (Bates et al. Reference Bates, Mäechler, Bolker and Walker2015; R Core Team 2015). Models had a binomial distribution and site was a random factor. Coefficients of variation were calculated using the R library goeveg (R Core Team 2015; Goral and Schellenberg Reference Goral and Schellenberg2017). Microclimate and climate normal variables were analysed through a linear regression model.
Results
During the 2015 and 2016 field seasons a total of 46 sites (31 in 2015 and 15 in 2016) were surveyed for Hesperiid butterflies; nine of these sites were positive Dakota skipper sites while the remaining 37 were negative sites. Vegetation and soil data were obtained for all 46 sites. Ground-level microclimate data were retrieved from 28 sites (five positive Dakota skipper sites and 23 negative sites). Climate normal data were obtained for all 46 sites from McKenney et al. (Reference McKenney, Hutchinson, Papadopol, Lawrence, Pedlar and Campbell2011). Representative site photographs taken during the study period are provided as Supplemental Figures 2–18.
Vegetation
There were no significant differences in plant composition between Dakota skipper positive and negative sites (F=0.6447; df=45; P=0.943) (Fig. 3A). The two-dimensional nonmetric multidimensional scaling has a final stress of 0.248. The first axis represents a gradient from plant communities dominated by Rumex crispus Linnaeus (Polygonaceae), Poa palustris Linnaeus (Poaceae), Trifolium hybridum Linnaeus (Fabaceae), and Hordeum jubatum Linnaeus (Poaceae) on the negative end to Lilium philadelphicum Linnaeus (Liliaceae), Juniperus horizontalis Moench (Cupressaceae), and Lygodesmia juncea (Pursh) Don ex Hooker (Asteraceae) on the positive end. The second axis represents a gradient from plant communities dominated by Poa palustris, Rumex Linnaeus (Polygonaceae) species, and Erigeron caespitosus Nuttall (Asteraceae) on the negative end to Symphyotrichum ericoides (Linnaeus) Nesom (Asteraceae), Asclepias ovalifolia Decaisne (Asclepiadaceae), Sonchus arvense Linnaeus (Asteraceae), and Cerastium nutans Rafinesque (Caryophyllaceae) on the positive end. All observed plant species are listed in Supplemental Table 1 and raw plant species site data are provided in Supplemental Table 2.
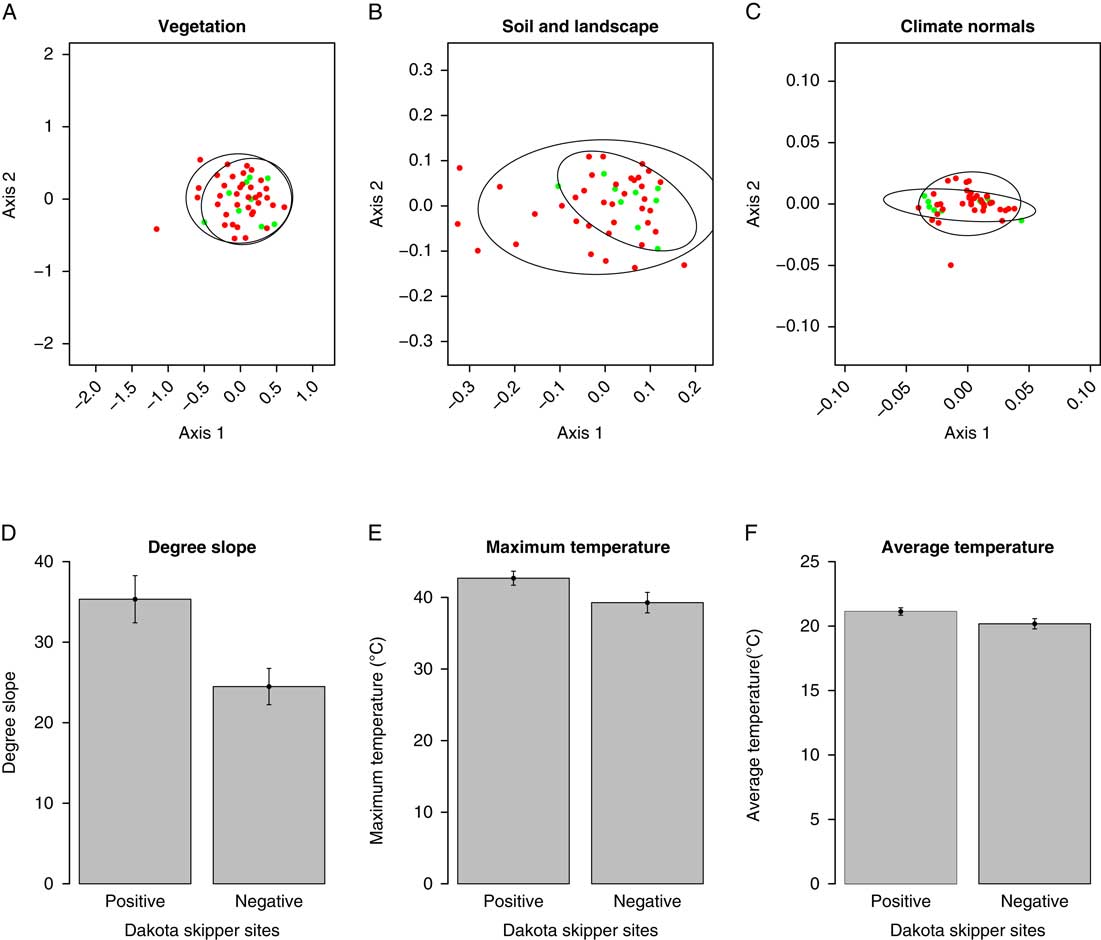
Fig. 3 Nonmetric multidimensional scaling ordinations of plant community composition (A), soil and landscape variables (B), and climate normal variables (C). Red dots indicate negative Dakota skipper occupancy and green dots indicate positive Dakota skipper occupancy. Bar graphs of significant variables degree slope (D), maximum temperature (E), and average temperature (F) with error bars representing standard error.
Indicator species analysis results identified three plant species that were significant indicators of Dakota skipper presence. Pediomelum argophyllum (Pursh) Grimes (Fabaceae) (IV=0.637; P=0.050) and Schizachyrium scoparium (Michaux) Nash (Poaceae) (IV=0.561; P=0.016) are common across all sites but more abundant in the Dakota skipper positive sites. Zizia aptera (Gray) Fernald (Apiaceae) (IV=0.207; P=0.038) is uncommon throughout the study area but more likely to be present in positive Dakota skipper sites. Full indicator species analysis results are provided in Supplemental Table 3. There were no significant indicator species for Dakota skipper negative sites.
Soil and landscape
There were no significant overall differences in soil and landscape variables between Dakota skipper positive and negative sites (F=1.253; df=45; P=0.223) (Fig. 3B). The two-dimensional nonmetric multidimensional scaling has a final stress of 0.175. The first axis represents a gradient from sites dominated by bulk density, per cent sand content, and per cent introduced species on the negative end to per cent silt content, degree slope, organic C, and per cent clay content on the positive end. The second axis represents a gradient from sites dominated by bulk density and per cent native prairie species on the negative end to per cent introduced species, litter depth, field-moist, and air-moist soil water content on the positive end. Full soils data are provided in Supplemental Table 5, and site landscape data are provided in Supplemental Table 6. Degree slope (P=0.045) was the only landscape variable significantly associated with Dakota skipper presence (Table 1); positive sites had a higher average slope of 35.33° while negative sites averaged 24.49° (Fig. 3D).
Table 1 Generalised linear model results of landscape and soil variables on Dakota skipper occupancy.
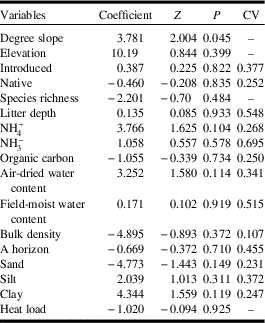
Z, z-value; P, P-value; CV, coefficient of variation.
Climate
There were no significant differences in overall climate conditions between Dakota skipper positive and negative sites (F=0.838; df=45; P=0.398) (Fig. 3C). The two-dimensional nonmetric multidimensional scaling has a final stress of 0.0287. The first axis represents a gradient from sites dominated by annual minimum temperature, October and April minimum temperatures on the negative end to November and March maximum temperatures and average precipitation on the positive end. The second axis represents a gradient from sites dominated by December, November, and January precipitation on the negative end to October, April, and annual minimum temperatures, and elevation on the positive end.
Microclimate
Maximum daily temperature and average daily temperature were significantly higher at Dakota skipper positive sites (Table 2). Dakota skipper positive sites had an average maximum daily temperature of 42.68 °C whereas negative sites had an average maximum daily temperature of 39.28 °C (Fig. 3E). Similarly, Dakota skipper positive sites had an average daily temperature of 21.13 °C, whereas negative sites had an average daily temperature of 20.18 °C (Fig. 3F). Minimum temperature was not significantly higher with Dakota skipper presence. While the climate normals are estimated at much coarser scale (10 km), there was a significant positive relationship between both maximum and minimum monthly temperature microclimate and the climate normals (Table 3).
Table 2 Generalised linear mixed model results of microclimate variables on Dakota skipper occupancy.
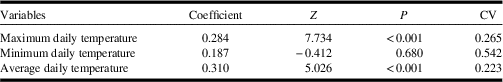
Z, z-value; P, P-value; CV, coefficient of variation.
Table 3 Linear regression model results of microclimate and climate normal maximum and minimum monthly temperatures.

Z, z-value; P, P-value.
Discussion
During the study period the Dakota skipper was observed at nine randomly selected sites throughout the Souris River Valley, adding to the previously known Saskatchewan populations (Hooper Reference Hooper2003; Webster Reference Webster2007; Westwood Reference Westwood2010). These results indicate that Dakota skipper populations are more prevalent within the Souris River Valley than initially thought. Dana (Reference Dana1991) states that the Dakota skipper requires a variety of native flora, which will vary in their contribution as nectaring sources. Results indicate that variation in native plant community composition does not appear to control Dakota skipper distribution, however three native plant species were significantly associated Dakota skipper presence including the forbs P. argophyllum and Z. aptera and native grass S. scoparium. Soil and landscape variables, with the exception of slope, were generally not good predictors of Dakota skipper detected occupancy. Slope was the only landscape variable with a significant relationship to detected occupancy of Dakota skippers, with populations tending to occur on steeper native prairie slopes. In addition, the Dakota skipper tends to inhabit locations that contain a warmer average and maximum daily microclimate within this region.
Dakota skipper presence is possible across a fairly wide range of vegetative community compositions, especially when plant species P. argophyllum, Z. aptera, and S. scoparium are present. Dakota skipper occupancy is significantly associated with native forb species P. argophyllum and Z. aptera. Ultimately, the Dakota skipper is a herbivore and requires native forbs for nectaring (Dana Reference Dana1991). Furthermore, Dakota skipper butterflies have been observed using native flora as perching platforms; Dana (Reference Dana1991) indicates that the Dakota skipper will perch on the tallest vegetation within a habitat while seeking a potential mate. During the study period P. argophyllum was prevalent on both positive and negative sites and was often the tallest forb within the site, making it an ideal perching platform for the Dakota skipper during the mating season. Although not found to be significant in previous studies (McCabe Reference McCabe1981; Dana Reference Dana1991, Reference Dana1997; Webster Reference Webster2007; Westwood Reference Westwood2010), these native forb species are important indicators of Dakota skipper populations of southeastern Saskatchewan as they are likely of value to the butterflies for both nectaring and mating activities.
The Dakota skipper is also significantly associated with native grass species S. scoparium. Layberry et al. (Reference Layberry, Hall and Lafontaine1998) and Webster (Reference Webster2007) note that S. scoparium is a host to Dakota skipper larvae. Additionally, Dana (Reference Dana1991) found that S. scoparium is a favoured native bunchgrass species used by Dakota skipper larvae for food and shelter. Native prairie bunchgrass species are necessary for Dakota skipper larvae survival as they are fine stemmed, close to the ground, and develop slower. Tame grass species tend to mature more quickly, are high off the ground, and tend to be overly hairy or smooth, making them unsuitable for larvae use. These characteristics of tame grass species inhibit the use of these grasses to Dakota skipper larvae while characteristics of native bunchgrass species enable Dakota skipper larvae to develop shelters and feed later into the season (Dana Reference Dana1991; Cochrane and Delphey Reference Cochrane and Delphey2002). Native prairie bunchgrass species are also used by the adult life form of the Dakota skipper (Webster Reference Webster2007); Westwood (Reference Westwood2010) observed female Dakota skipper ovipositing on S. scoparium, and eggs can be found on these same bunchgrass species (Dana Reference Dana1991). The United States Fish and Wildlife Service (2015) states that Dakota skipper success will greatly depend on the presence and development of these bunchgrass species as both larvae and adult life forms of this species use them.
Soil and landscape variables were found to overlap between positive and negative Dakota skipper sites, suggesting they are generally not good predictors of Dakota skipper presence within the Souris River Valley, Saskatchewan. However, significant differences in per cent slope between positive and negative sites suggest that Dakota skippers may prefer sites on significantly steeper south (sites 18; Dakota skipper observations 3; proportion 0.17), east (sites 8; Dakota skipper observations 2; proportion 0.25), and west (sites 12; Dakota skipper observations 4; proportion 0.35) facing slopes opposed to north (sites 2; Dakota skipper observations 0; proportion 0) facing slopes. This is consistent with the Webster (Reference Webster2007) Dakota skipper population observations within this region, all of which were on steep south-facing slopes. The Dakota skipper is an ectothermic species that requires heat for development and Saskatchewan Dakota skipper populations are at the extreme northern extent of their distribution (Committee on the Status of Endangered Wildlife in Canada 2014); these south-facing and west-facing slopes contain a warmer microclimate that may be needed for Dakota skipper larval development (Weiss and Weiss Reference Weiss and Weiss1998).
Climate normal variables were found to overlap in positive and negative Dakota skipper sites indicating that climate normals are generally not good predictors of Dakota skipper presence within the Souris River Valley. Past research indicates that Dakota skipper distribution may be influenced by climate factors including temperature, humidity (McCabe Reference McCabe1981; Royer et al. Reference Royer, McKenney and Newton2008; Dearborn and Westwood Reference Dearborn and Westwood2014), and precipitation–evaporation ratios which affect larvae development (McCabe Reference McCabe1981; Royer et al. Reference Royer, McKenney and Newton2008). However, Turner et al. (Reference Turner, Gatehouse and Corey1987) found that these climate patterns are observed at the microclimate of the habitat of a butterfly.
Ground-level maximum and daily average temperatures were higher at Dakota skipper positive sites in southeastern Saskatchewan compared with Dakota skipper negative sites. This is likely due to the Dakota skipper being an ectothermic species that requires heat to develop and reach maturity (Dearborn and Westwood Reference Dearborn and Westwood2014). Southern Saskatchewan is at the northwestern edge of Dakota skipper range; these results suggest that the Dakota skipper may be limited to warmer than average sites in this region. Minimum growing season daily temperatures were not a significant indicator of Dakota skipper habitat, however it is possible that higher minimum winter temperatures would also be significantly associated with Dakota skipper presence. Ehrenreich and Aikman (Reference Ehrenreich and Aikman1963) state that increased litter and snow cover provides insulation to Dakota skipper larvae that spend the winter months in the upper soil layers, protecting them from extreme cold temperatures. The extreme cold temperatures and limited snow cover common in this region may limit overwinter larval survival in southeastern Saskatchewan.
Climate normals and the microclimate maximum and minimum monthly temperatures are significantly related to one another, indicating there is a relationship between climate normals and microclimate. This indicates that climate normals are a good proxy for microclimate conditions, suggesting that climate normals can be used as predictors of Dakota skipper habitat. This is important, as mapped climate normal values are available for large-scale modelling of potential Dakota skipper habitat.
In conclusion, the Dakota skipper populations of southeastern Saskatchewan appear to be limited to native prairie containing significant vegetative species, steep landscape slopes, and a warm microclimate. These results indicate that Dakota skipper populations are possible on a variety of sites within southeastern Saskatchewan given the presence of appropriate vegetation on the correct landscape positions that contain a warmer microclimate. Additional Dakota skipper populations are likely present in southern Saskatchewan; further research focussed on modelling and mapping potential habitat in this region is underway. Targeted survey efforts focussed in this potential habitat is important to fully evaluate the conservation status of the Dakota skipper. As we begin to understand this species habitat associations we can begin to develop best management techniques (Layberry et al. Reference Layberry, Hall and Lafontaine1998; Environment Canada 2007; Webster Reference Webster2007; Committee on the Status of Endangered Wildlife in Canada 2014).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.4039/tce.2018.33










