Introduction
The fall armyworm, Spodoptera frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae), is currently considered one of the most important agricultural pests in the main commodity-producing regions of the world (Goergen et al., Reference Goergen, Kumar, Sankung, Togola and Tamò2016; Bateman et al., Reference Bateman, Day, Luke, Edgington, Kuhlmann and Cock2018; Sun et al., Reference Sun, Hu, Jia, Wu, Shen, Zhao, Jiang and Wu2021). In the Brazilian agricultural landscape, S. frugiperda stands out for its very high biotic potential and generates approximately eight generations per year (Montezano et al., Reference Montezano, Specht, Sosa-Gómez, Roque-Specht, Paula-Moraes, Peterson and Hunt2019). In addition, this species has a high capacity for dispersion, migration, and polyphagia, which favors their occurrence in different host plants of economic importance (for example maize, soybean, and rice) (Montezano et al., Reference Montezano, Specht, Sosa-Gómez, Roque-Specht, Paula-Moraes, Peterson and Hunt2019).
In Brazil and other places where S. frugiperda occurs, the main strategies used for its management in corn crops are applications of synthetic insecticides (e.g., pyrethroids, diamides, spinosyns, carbamates, and organophosphates, among others) (Carvalho et al., Reference Carvalho, Omoto, Field, Williamson and Bass2013; Burtet et al., Reference Burtet, Bernardi, Melo, Maiquel, Strahl and Guedes2017). Additionally, genetically modified plants express insecticidal proteins from Bacillus thuringiensis Berliner (Bt) (Bernardi et al., Reference Bernardi, Malvestiti, Dourado, Oliveira, Martinelli, Berger, Head and Omoto2012; Yano et al., Reference Yano, Specht, Moscardi, Carvalho, Dourado, Martinelli, Head and Sosa-Gómez2015; Marques et al., Reference Marques, Santos, Castro, Moscardini, Rossetto, Silva, Zobiole, Valverde-Garcia, Babcock, Storer, Rule and Fernandes2017). However, inappropriate use of these technologies has contributed to the rapid evolution of S. frugiperda resistance to insecticides (Diez-Rodríguez and Omoto, Reference Diez-Rodrígues and Omoto2001; Carvalho et al., Reference Carvalho, Omoto, Field, Williamson and Bass2013; Nascimento et al., Reference Nascimento, Farias, Bernardi, Horikoshi and Omoto2016; Okuma et al., Reference Okuma, Bernardi, Horikoshi, Bernardi, Silva and Omoto2018; Bolzan et al., Reference Bolzan, Padovez, Nascimento, Kaiser, Lira, Amaral, Kanno, Malaquias and Omoto2019) and Bt proteins (Bernardi et al., Reference Bernardi, Bernardi, Horikoshi, Okuma, Miraldo, Fatoretto, Medeiros, Burdc and Omoto2016; Horikoshi et al., Reference Horikoshi, Bernardi, Bernardi, Malaquias, Okuma, Miraldo, Amaral and Omoto2016). This scenario has prompted new studies aimed at developing new strategies and approaches, as well as alternative methods for the management of fall armyworm (Paredes-Sánchez et al., Reference Paredes-Sánchez, Rivera, Bocanegra-García, Martínez-Padrón, Berrones-Morales, Niño-García and Herrera-Mayorga2021).
Using products that have low toxicity to beneficial insects and nontarget organisms and have reduced environmental impact are important strategies to be incorporated into integrated pest management (IPM) programs (Dhadialla et al., Reference Dhadialla, Carlson and Le1998; Lira et al., Reference Lira, Wanderley-Teixeira, Teixeira, Cunha, Cruz and Neto2020). In this context, insecticides that regulate insect growth (IRGs) may play an important role in the management of S. frugiperda (Toscano et al., Reference Toscano, Calado Filho, Cardoso, Marayama and Tomquelski2012), including both those of synthetic and natural origins.
Among products with natural origins, botanical insecticides have expanded their market significantly in recent years (Isman, Reference Isman2020). However, their contribution is still small compared to that of synthetic insecticides. Botanical insecticides are produced from substances produced by plant secondary metabolisms (allelochemicals); they generally have a broad spectrum of action against pest arthropods and may cause lethal toxicity or behavioral changes, such as repellency and deterrence of feeding and oviposition (Ribeiro et al., Reference Ribeiro, Vendramim, Gonçalves, Ansante, Gloria, Lopes, Mello-Silva and Fernandes2016; Bernardi et al., Reference Bernardi, Bernardi, Horikoshi, Salmeron, Okuma, Farias, Nascimento and Omoto2017; Tak and Isman, Reference Tak and Isman2017; Geisler et al., Reference Geisler, Martins, Treptow, Baronio, Stupp, Ribeiro, Garcia and Bernardi2019). In addition, the rapid degradation of these compounds in the environment (photodegradable) causes these products to be easily accepted by the community as safer and more sustainable (Amoabeng et al., Reference Amoabeng, Gurr, Gitau and Stevenson2014; Isman, Reference Isman2020).
Among the most well-known and widespread botanical insecticides are those produced from neem, Azadirachta indica (Juss) (Meliaceae), whose main active ingredient is the tetranortriterpenoid, azadirachtin (Campos et al., Reference Campos, Oliveira, Pascoli, Lima and Fraceto2016; Isman, Reference Isman2020). Azadirachtin may exert anti-feeding (acting on insect chemoreceptors) and repellent actions (Isman, Reference Isman2006; Qin et al., Reference Qin, Zhang, Zhou, Liu, Xiao, Chen and Zhang2019). In addition, it can interfere with the development of endocrine glands that control insect metamorphosis by compromising the ecdysis process (Mordue (Luntz) and Blackwell, Reference Mordue (Luntz) and Blackwell1993). Studies have demonstrated high acute lethal toxicity levels of this compound on arthropod-pests with different feeding habits, such as leafminers (fly and moth larvae); suckers (mites, thrips, aphids, and bed bugs); and chewers (larvae and scarabs) (Adhikari et al., Reference Adhikari, Bhandari, Niraula and Shrestha2020), including effects on larvae and adults of S. frugiperda (Duarte et al., Reference Duarte, Redaelli, Jahnke and Trapp2019). Despite these promising bioactive effects, the use of IRGs has not been widespread in IPM programs due to the incessant search for products with fast action (knock-down effects, especially) in pest control (Singh and Suri, Reference Singh and Suri2013).
In addition to their lethal effects, neem-based insecticides and growth regulators of synthetic origin can produce sublethal effects at certain concentrations, such as increases in larval and pupal periods, reductions in pupal weight and viability and increases in the percentage of adults with malformations (Lima et al., Reference Lima, Oliveira, Gondim, Marques and Correia2010; Roel et al., Reference Roel, Dourado, Matias, Porto, Bednaski and Costa2010; Duarte et al., Reference Duarte, Redaelli, Jahnke and Trapp2019). These effects can directly influence insect population dynamics in future generations and favor management strategies (Cabodevilla et al., Reference Cabodevilla, Ibañez, Simón, Murillo, Caballero and Williams2011). Based on this assumption, the objective of this study was to evaluate the sublethal effects of growth regulating insecticides of synthetic and botanical origins on the biological parameters and fertility life table of S. frugiperda, under controlled laboratory conditions.
Material and methods
Tested insects
Specimens used in the tests came from a population of S. frugiperda collected from conventional (non-Bt) corn during the 2012/2013 season in Mogi Mirim, São Paulo, Brazil (22°28′31″ S and 46°54′21″ W). In the laboratory, the larvae were maintained for more than 25 generations on an artificial diet (Kasten et al., Reference Kasten, Precetti and Parra1978). For adult feeding, a 10% (v.v−1) honey solution was used. The moths were kept in cylindrical PVC cages (24.0 cm height × 14.5 cm diameter), which were internally lined with newsprint as a substrate for oviposition, and closed at the top with voile fabric.
Concentration-response curves
To characterize the concentration-response curves for each product (Table 1), bioassays using the method of incorporating an artificial diet as proposed by the Insecticide Resistance Action Committee (Method 20, IRAC International) were used (IRAC 2011). For this purpose, six concentrations of each insecticide (range: 312.5–10,000 mg kg−1), diluted in distilled water, were used (IRAC 2011). As a negative control, distilled water was used. For the bioassays, we used the artificial diet proposed by Kasten et al. (Reference Kasten, Precetti and Parra1978), which is commonly used for rearing S. frugiperda. The insecticide concentrations were incorporated into the diets at temperatures between 45 and 50°C, with the aid of a vortex-type shaker for 2 min. Subsequently, the diets were distributed in 16-well plastic plates (Advento do Brasil, São Paulo, Brazil) (4 ml per well). After cooling the diets, one S. frugiperda neonate larva (0–24 h old) was added to each well using a fine brush. The plates were closed and maintained in a chamber at 25 ± 1°C, 60 ± 10% RH, and a photoperiod of 14:10 (L:D) h. For each concentration, 8 repetitions (wells) were used, each repetition consisted of 5 larvae, totaling 80 larvae per concentration. Larval mortality was evaluated at 7 days. Larvae that survive beyond the first instar were also considered dead.
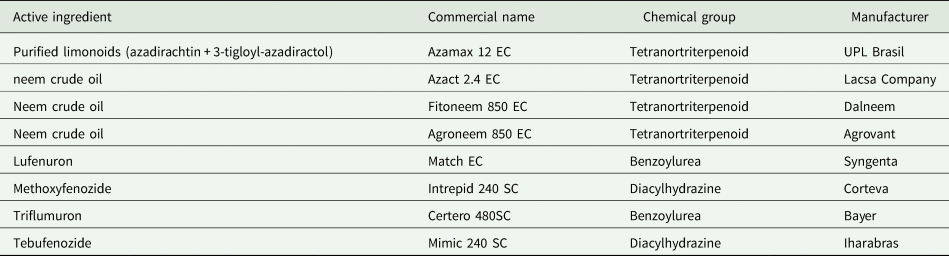
Table 1. Botanical and synthetic growth regulating insecticides evaluated against Spodoptera frugiperda
Sublethal effects insecticides on Spodoptera frugiperda
To evaluate the sublethal effects of the insecticides on S. frugiperda, LC25 and LC50 concentration of each insecticide (Table 2) were used in bioassays when incorporated in artificial diets (IRAC 2011). For this, the defined concentrations were added to the artificial diet as described by Kasten et al. (Reference Kasten, Precetti and Parra1978) when the temperature of the diet reached 45–50°C. The incorporations were performed with a vortex tube shaker for 2 min. Diets containing the incorporated products were distributed in glass containers (8.0 cm height × 2.4 cm diameter, with a total volume of 64.84 cm2) containing 4 ml diet tube−1 with the aid of a repeat dosing pipette. After gelling and cooling the diets and with the aid of a fine brush, neonate S. frugiperda larvae were inoculated (1 larva < 24 h age) in each tube. The tubes were sealed with hydrophilic cotton to allow gas exchange with the external environment, and kept in a climatized room (temperature 25 ± 1°C, relative humidity 60 ± 10% and photophase 14:10 h L:D). For each insecticide concentration (LC25 or LC50), 10 replicates (tubes) were used, with 16 larvae/repetition, totaling 160 larvae per insecticide.
Table 2. Concentration-mortality response (LC; g or ml of commercial product) of S. frugiperda neonates exposed to the artificial diet treated with different concentrations of botanical and synthetic growth regulating insecticides

a LC25 and LC50: concentration of commercial product (g or ml per 100 l−1 of water) required to kill 25 and 50% of larvae in the observation period of 7 days, respectively.
b LC25 and LC50 values designated by different letters within a column are significantly different from each other through non-overlap of 95% fiducial limits. Significance of differences among slopes determined by likelihood ratio test of equality followed by pairwise comparisons using non-overlapping fiducial limits.
c χ2 significant.
d Degrees of freedom.
The following biological parameters of the immature stages were evaluated: survival (%) at 7 days after infestation (d.a.i) and survivorship at adult eclosion, duration (days) of neonate-to-adult eclosion, larval weight (mg) at 7 d postinfection (d.p.i.), and pupal weight (mg) 24 h after pupal formation. The total fecundity (number of total eggs per female) was evaluated by forming 20 couples/treatment of S. frugiperda, which were paired separately in PVC cages (23 cm height × 10 cm diameter), which were internally coated with white paper and closed with sheer fabric. The number of eggs was counted daily and the mortality of adults was recorded.
Statistical analyses
To estimate the lethal concentrations (LC25 and LC50) and respective CIs, the concentration-mortality data of each product were subjected to Probit analysis (PROC PROBIT, SAS® Institute 2000, Cary, NC). A likelihood ratio test was conducted to test the hypothesis that the LC values were equal. If the hypothesis was rejected, pairwise comparisons were performed, and significance was declared if the CIs did not overlap (Robertson et al., Reference Robertson, Russell, Preisler and Savin2007). To evaluate the sublethal effects of the insecticides on S. frugiperda receiving artificial diets, data from all biological parameters evaluated [survival at 7 d.a.i and survivorship at adult eclosion (%), duration of neonate-to-adult eclosion, larval weight, pupal weight and total fecundity of S. frugiperda] were evaluated for normality by the Shapiro-Wilk test and homoskedasticity by the Hartley and Bartlett test. Subsequently, data were subjected to a two-way ANOVA using the PROC GLM procedure in SAS® 9.1 (SAS Institute, 2011). Factor A was represented by the insecticide concentrations (LC25 or LC50). Factor B was composed of insecticides (e.g., Certero, Match, Intrepid, Mimic, Azamax, Agroneem, Azact and Fitoneem) and untreated controls. The insecticide concentrations and interactions were used as fixed factors in the model. Mean differences were calculated by the Least-Square Means Statement (LSMEANS option of PROC GLM) using a Tukey-Kramer adjustment test (P < 0.05) in SAS® 9.1 (SAS Institute, 2011).
Survivorship, development time, and reproductive data were used to estimate the population growth parameters, such as the mean length of one generation (T), net reproductive rate (Ro; average number of female offspring that would be born to a cohort of females), and intrinsic rate of population increase (rm; daily production of females per parental female). Fertility life table parameters were obtained using the jackknife technique by applying the ‘lifetable.sas’ procedure developed by Maia et al. (Reference Maia, Luiz and Campanhola2000) in SAS® 9.1 (SAS Institute, 2011).
Results
Concentration-response curves
Overall, S. frugiperda larvae were more susceptible to growth-regulating insecticides than compared to neem-based insecticides (Table 2). When comparing the susceptibilities to the neem-based insecticides, it was observed that Azact was more toxic to larvae and had the lowest LC25 and LC50 values (LC25 = 102.4 and LC50 = 210.5 – Table 2) compared to Agroneem (LC25 = 653.7 and LC50 = 1,115.3), Azamax (LC25 = 271.3 and LC50 = 700.5) and Fitoneem (LC25 = 125.2 and LC50 = 198.3) (Table 2). The values for the growth-regulating insecticides Certero (LC25 = 37.8 and LC50 = 69.4 – Table 2), relative to the growth-regulating insecticides, Intrepid (LC25 = 66.9 and LC50 = 134.7), Match (LC25 = 89.4 and LC50 = 165.4) and Mimic (LC25 = 43.2 and LC50 = 74.5) (Table 2) were verified.
Survivorship
The larval survival of S. frugiperda neonates was significantly affected from the use of the LC25 or LC50 concentration values of the products at 7 d.p.i. (LC25: F = 96.0; df = 8, 81; P < 0.0001 and LC50: F = 84.1; df = 8, 81; P < 0.0001, respectively). Azamax 12 EC caused the lowest larval survival of S. frugiperda at 7 d.a.i, which was similar to the results for growth-regulating insecticides of synthetic origin (e.g., Certero 480 SC, Match EC, Intrepid 240 SC, and Mimic 240 SC) at the LC25 (variations in survival between 54.2 to 59.6%) and LC50 (variations in survival between 51.1 to 55.3%) concentrations (Table 3). In contrast, for the other products of botanical origin (e.g., Agroneem 850 EC, Azact 1.4 EC, and Fitoneem 850 EC), neonate survival was equal to or greater than 80% at LC25 and above 66% at the LC50 concentration (Table 3). In the same way, there were significant interactions between the artificial diets and concentrations evaluated (LC25 or LC50) (F = 112.9; df = 8, 81; P < 0.0001 and F = 111.3; df = 8, 81; P < 0.0001) regarding survivorship at 7 d (fig. 1a) and for survivorship at adult eclosion for all products (fig. 1b). However, Azamax produced the lowest rates of survival to adulthood for both the LC25 (46.2%) and LC50 (42.2%) concentrations, which were statistically similar to treatments with growth-regulating insecticides of synthetic origin (Table 3).

Fig. 1. Survivorship (a), Survivorship at adult eclosion (b), neonate-to-adult eclosion period (c), larval weight (d), pupal weight (e) and number of eggs/female (f) of S. frugiperda developing on artificial diet treated with different concentrations of botanical and synthetic growth regulating insecticides. Pairs of bars (± SE) with the same letter are not significantly different (LSMEANS followed by Tukey test; P > 0.05).
Table 3. Survivorship (% ± SE) of S. frugiperda neonates developing on artificial diet treated with different concentrations of botanical and synthetic growth regulating insecticides

a Means ± SE within a column followed by the same letter in each at concentration evaluated are not significantly different (LSMEANS followed by Tukey-Kramer test; P > 0.05).
b LC25 or LC50 concentrations used against S. frugiperda neonates.
Development
Regardless of the product or concentration tested (LC25 or LC50), incorporation of insecticides based on limonoids extracted from neem and growth-regulating insecticides of synthetic origin in an artificial diet resulted in a significant increase in duration of the larval stage and the duration (neonate to adult) of S. frugiperda compared to the negative control (diet only) (Table 4). The botanical insecticide, Azamax, was associated with the longest duration (longer than 70 days) of the neonate to adult period in relative to all other treatments, regardless of the concentration used (Table 4 – LC25: F = 96.8; df = 8, 119; P < 0.0001 or LC50: F = 69.4.82; df = 8, 119; P < 0.0001). However, larvae fed crude neem oil (e.g., Agroneem, Azact, and Fitoneem) had shorter neonate to adult periods (~46 days for LC25 and ~56 days for LC50 concentrations) (Table 4). However, S. frugiperda larvae fed artificial diets treated with growth-regulating insecticides of synthetic origin from the benzoylurea (e.g., Certero and Match) and diacylhydrazine (e.g., Intrepid and Mimic) groups had neonate-to-adult durations of ~52 to 57 days at LC25 and ~ 60 days at LC50 concentrations (Table 4). In turn, larvae fed untreated diets (negative control) had developmental periods of 30 days (Table 4). The neonate-to-adult periods for larvae exposed to LC50 concentrations were consistently longer than those for larvae fed artificial diets treated with LC25 concentrations for all products evaluated (fig. 1c). The weights of larvae (7 days) were also significantly affected when neem-based products or synthetic growth regulators were added to the artificial diet (Table 4). The lowest larval weights were observed in treatments consisting of products of synthetic origin (LC25 or LC50) (Table 4 – LC25: F = 117.12; df = 8, 84; P < 0.0001; LC50: F = 99.4; df = 8, 80; P < 0.0001). By comparing the results when LC25 and LC50 concentrations were used in the artificial diets it was found that larvae fed artificial diets containing LC50 insecticide concentrations consistently had significantly lower weights (fig. 1d). In addition, pupal weights (24 h) were also significantly affected by all treatments tested relative to the control treatment (Table 4 – LC25: F = 103.4; df = 8, 81; P < 0.0001; LC50: F = 117.9; df = 8, 81; P < 0.0001). The surviving larvae fed artificial diets treated with LC50 concentrations of the insecticides produced pupae with lower weights than those that developed with LC25 concentrations (fig. 1e). However, there were no significant differences among insecticides based on limonoids extracted from neem and growth-regulating insecticides of synthetic origin (Table 4).
Table 4. Biological parameters (mean ± SE) of S. frugiperda developing on artificial diet treated with different concentrations of botanical and synthetic growth regulating insecticides

a Means ± SE within a column followed by the same letter in each at concentration evaluated are not significantly different (LSMEANS followed by Tukey-Kramer test; P > 0.05).
b LC25 or LC50 concentrations used against S. frugiperda neonates.
Reproduction and population growth
Regardless of the concentration used (LC25 or LC50) and product tested, S. frugiperda females receiving such treatments showed lower total fecundities (number of eggs/female) in relation to control (Table 4 – LC25: F = 169.8; df = 8, 136; P < 0.0001; LC50: F = 197.3; df = 8, 136; P < 0.0001). Greatest reductions were observed in females from larvae fed an artificial diet content benzoylurea triflumuron (Certero) in LC25 or LC50 concentrations. However, females from larvae that developed on artificial diets treated with the LC50 concentration had significantly lower fecundities than those exposed to the LC25 concentrations (fig. 1f).
According to the estimated life table parameters (Table 5), all larvae maintained on artificial diets containing growth-regulating insecticides of synthetic and botanical origins showed significant effects on their fertility life table parameters compared to the negative control (Table 5). The botanical insecticide, Azamax caused the greatest increases in duration of each generation, which were (T) ~75 and 80.9 d when the larvae were exposed to artificial diets treated with LC25 or CL50 concentrations respectively. In addition, fewer than ~84.8 and 45.4 females/newborn female/generation (Ro) were produced, respectively, whereas the progeny survivors in the control group produced approximately 250 females/female over ~36 d (Table 5). The female progeny of the survivors of the diet containing Azamax exhibited natural population increase rates (rm) lower than 0.063 and 0.045 on LC25 at LC50 concentrations respectively, indicating an approximately 60 to 70% lower capacity for population increase when compared to the control treatment (Table 5). Certero, Intrepid, Match and, Mimic at LC25 and LC50 concentrations also showed significant reductions in the Ro and rm values relative to the control treatment (Table 5).
Table 5. Fertility life table parameters of Spodoptera frugiperda developing on artificial diet treated with sublethal concentrations (LC25 or LC50) of different growth regulating insecticides of synthetic and botanical origin

a T, mean length of a generation (d); Ro, net reproductive rate (females per female per generation); and rm, intrinsic rate of population increase (per day).
b Means ± SE within a column followed by the same letter in each concentration (LCs) are not significantly different (t-test for pairwise group comparisons, P > 0.05).
In all comparisons between artificial diets treated with LC25 insecticide concentrations and diets with LC50 insecticide concentrations, the population growth parameters were always lower for artificial diets treated with LC50 levels (Table S1). These findings indicate that the more pronounced sublethal effects of insecticides at LC50 levels provide a greater population suppression of S. frugiperda over time.
Discussion
The results obtained in the present study demonstrated the potential sublethal effects of growth-regulating insecticides of botanical and synthetic origins on the developmental parameters of S. frugiperda. Additionally, this study demonstrated the potential for using these insecticides as an alternative strategy to manage this important pest species. The main bioactive effects observed were on the viability of the developmental stages (larval and pupal) and duration of the biological cycle (egg-adult). These aspects can considerably influence the population dynamics and demographic growth of the studied pest species under field conditions. This fact is already demonstrated by the estimated parameters of the life table and fertility.
Limonoid-rich neem-derived products (especially azadirachtin) have multiple modes of action against target species (Isman, Reference Isman2020). In addition to direct action on the production and release of ecdysteroids in hemolymph, the insecticide also blocks phago-stimulating cells, which leads to inhibition of feeding and delayed development (Mordue (Luntz), Reference Mordue (Luntz), Koul and Wahab2004; Duarte et al., Reference Duarte, Redaelli, Jahnke and Trapp2019). In turn, the effects caused by growth-regulating insecticides of synthetic origin are based, in the case of benzoylureas, on interrupting the formation of structures composed of chitin, which consequently leads to abnormal endocuticular deposition (Mulder and Gijswijt, Reference Mulder and Gijswijt1973). On the other hand, diacylhydrazines act as agonists of ecdysteroid receptors accelerating the molting process and by preventing satisfactory replacement of the old integument (Carlson et al., Reference Carlson, Dhadialla, Hunter, Jansson, Jany, Lidert and Slawecki2001). Although the effects of such products are already widely reported, our study indicates for the first time the effects of such products at sublethal concentrations on the fertility life table parameters. These parameters are important indicators of the population growth of a pest species under field conditions.
Among all botanical insecticides tested, Azamax was the most promising product, which stems from its higher azadirachtin and 3-tigloilazadiractol contents (previously azadirachtin A and B), which are considered the main active components of neem formulations (Isman, Reference Isman2017). On the other hand, the other neem products tested, notably crude oils with very diversified limonoid profiles, showed promising effects but to lesser extents when compared to Azamax.
By incorporating the tested insecticides into the diets, it was possible to visually observe reductions in diet intakes and consequently, weight decreases in larvae and pupae. Feeding reductions directly affect the critical weights of exposed organisms (Nijhout et al., Reference Nijhout, Riddiford, Mirth, Shingleton, Suzuki and Callier2014), as reported in studies by Duarte et al. (Reference Duarte, Redaelli, Jahnke and Trapp2019), which corroborates the results found in the present study. Roel et al. (Reference Roel, Dourado, Matias, Porto, Bednaski and Costa2010) reported that during metamorphosis process, insects do not fully utilize the nutrients contained in their diets, so the larvae do not gain weight before juvenile hormones are depleted and ecdysteroid levels increase and consequently, smaller pupae result. However, Lima et al. (Reference Lima, Oliveira, Gondim, Marques and Correia2010) did not observe reductions in pupal weight, a fact that must be related to the shorter exposure time (48 h) of the larvae to neem derivatives in that study, which made it impossible to find potential postingestive effects.
Low levels of nutritional reserves increase the duration of the pupal period, as nutrients support successful metamorphosis, directly influencing the reproduction and survival of individuals (Merkey et al., Reference Merkey, Wong, Hoshizaki and Gibbs2011). Toscano et al. (Reference Toscano, Calado Filho, Cardoso, Marayama and Tomquelski2012) reported increased mortality of S. frugiperda larvae exposed to neem and lufenuron compared to pyroligneous extracts. Assimilation of nutrients during the larval period directly affects fecundity (Arrese and Soulages, Reference Arrese and Soulages2010). In the present study, significant reductions in total fertility were observed when comparing the control group with the other treatments. Research involving the genus Spodoptera found an association between small-sized pupae and fecundity rates (Specht et al., Reference Specht, Montezano, Sosa-Gómez, Paula-Moraes, Roque-Specht and Barros2015; Duarte et al., Reference Duarte, Redaelli, Jahnke and Trapp2019), reducing longevity (Pineda et al., Reference Pineda, Martínez, Figueroa, Schneider, Del Estal, Viñuela, Gómez, Smagghe and Budia2009; Lima et al., Reference Lima, Oliveira, Gondim, Marques and Correia2010), with a significant impact on pest demographics in the field as well as on mating timing (Duarte et al., Reference Duarte, Redaelli, Jahnke and Trapp2019). In our study, we observed that the duration of each generation for all insecticides was longer compared to the negative control. However, we emphasize that the net reproduction rates were lower, thus causing smaller offspring.
Spodoptera exigua larvae (Hübner, 1808) (Lepidoptera: Noctuidae) fed methoxyfenozide and lufenuron exhibited reductions in larval and pupal weights, prolongation of larval and pupal development, and significant reductions in fertility, which also corroborated the results obtained in the present study (Chein et al., Reference Chein, Li, Wang, Liu, Mao, Chen and Zhang2019). Likewise, several authors report reductions in pupal weight and increases in larval and pupal development times for lepidopterans exposed to hexaflumuron, diflubenzuron, flufenoxuron and bistrifluron (El-Ghar et al., Reference El-Ghar, Radwan, El-Bermawy and Zidan2010; Mahmoudvand et al., Reference Mahmoudvand, Abbasipour, Garjan and Bandani2011; Zhu et al., Reference Zhu, He, Yao, Liu, Tao and Huang2012; Hafeez et al., Reference Hafeez, Li, Yousaf, Khan, Imran, Zhang, Huang, Zhang, Shah, Wang, Fernández-Grandon, Ali and Lu2021). These results demonstrate that S. frugiperda larvae exposed to neem-based insecticides or synthetic growth regulators exhibited suppression of large populations of this pest over time. The results indicate that neem-derived products, as well as growth-regulating insecticides of synthetic origin, cause sublethal effects on S. frugiperda, reducing their ability to reproduce and contributing to integrated management strategies that aim to keep populations below damaging levels. Thus, these insecticides are considered as environmentally safer alternatives for managing S. frugiperda in different agricultural production systems.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S000748532200058X
Acknowledgement
The authors would like to thank to the Coordination for Perfecting Higher Education Personnel (CAPES). The Rio Grande do Sul Research Support Foundation (FAPERGS) for the financial support (Process #19/2551-0001754-5) and Scientific Development (CNPq) for the productivity scholarship (Process 305377/2019-1 and 304018/2019-8) provided the last two authors, respectively.
Author contribution
LNM: Conceptualization, Methodology, Investigation, Data Curation, Writing – Original Draft. FCSG, MBA and DTTA: Investigation, Writing – Review & Editing. MR: Formal Analysis, Data Curation, Writing – Review & Editing. APSAdR and LPR: Resources, Supervision, Funding acquisition, Writing – Review & Editing. DB: Resources, Supervision, Project administration, Funding acquisition, Writing – Review & Editing.
Conflicts of interest
The authors declare no competing interests.









