Introduction
The red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae), is one of the most harmful stored-product secondary insect pests (Rees, Reference Rees2004; Mahroof and Hagstrum, Reference Mahroof, Hagstrum, Hagstrum, Philips and Cuperus2012). The timely discovery of its presence and abundance is an important preventive measure in successful control (Gorham, Reference Gorham and Gorham1991; Rees, Reference Rees2004; Mason and McDonough, Reference Mason, McDonough, Hagstrum, Philips and Cuperus2012). T. castaneum spreads within closed environments by moving across surfaces, and rarely by flying short distances (Arbogast, Reference Arbogast and Gorham1991). Movement depends on environmental stimuli, which may attract or repel the insects. Odour is one of the most important stimuli. Olfactory organs in the insects receive air-borne chemical stimuli (odours) that affect their behaviour (Su et al., Reference Su, Menuz and Carlson2009; Phillips and Throne, Reference Phillips and Throne2010) and lead them to food sources (Ajayi et al., Reference Ajayi, Balusu, Morawo, Zebelo and Fadamiro2015; Arnold et al., Reference Arnold, Stevenson and Belmain2015), partners (Tewari et al., Reference Tewari, Jyothi, Kasana, Prasad and Prasuna2015) or places suitable for laying eggs (Barrer and Jay, Reference Barrer and Jay1980). Food odour is an evaporable substance or a mixture of substances emanated from a food source and spread by air (Olsson, Reference Olsson2001).
Different methods of grain processing and other factors that result in grain damage most often increase commodity attractiveness to secondary pests (Freedman et al., Reference Freedman, Mikolajczak, Smith, Kwolek and Burkholder1982; Bergerson and Wool, Reference Bergerson and Wool1987; Trematerra et al., Reference Trematerra, Sciarreta and Tamasi2000; Campbell, Reference Campbell2012; Trematerra et al., Reference Trematerra, Ianiro, Athanassiou and Kavallieratos2015). For example, Seifelnasr et al. (Reference Seifelnasr, Hopkins and Mills1982) found in an olfactometer test that T. castaneum were significantly more attracted to volatiles of wheat grain parts (endosperm, germ), wheat flour and wheat bran than to the odour of whole wheat grain. Extracts of wheat germ were also more attractive than were the extracts of other fractions, while whole millet flour or fermented millet flour volatiles were more attractive than those from whole millet kernels or millet starch. The odours of whole grain may attract secondary pests when damaged kernels are not offered (Seifelnasr et al., Reference Seifelnasr, Hopkins and Mills1982; Ahmad et al., Reference Ahmad, Daglish, Ridley and Walter2012). Food attractiveness may be enhanced by secretions that insects leave behind while feeding, i.e. primary pest feeding may increase the attractiveness of substrates to secondary pests by inducing odour in damaged grain that is preferred by primary pests (Trematerra et al., Reference Trematerra, Sciarreta and Tamasi2000, Reference Trematerra, Ianiro, Athanassiou and Kavallieratos2015). The presence of dead and live adults in food considerably affects the attractiveness of food odour to secondary pests (Athanassiou et al., Reference Athanassiou, Kavallieratos and Campbell2015; Stevenson et al., Reference Stevenson, Cai, Faucher, Michie, Berna, Ren, Anderson, Chyb and Xu2017). When the synthetic aggregation pheromone, which is typical of the genus Tribolium, is added to food it also increases the attractiveness of its odours to species of the genus. It is more attractive to insects than the odours of food or pheromone alone (Phillips, Reference Phillips1997; Campbell, Reference Campbell2012; Dissanayaka et al., Reference Dissanayaka, Sammani and Wijayaratne2018).
The damage caused by stored-product insects in the feed industry is great, but research efforts have so far mostly focused on their harmfulness in human food industries. Consequently, there are little available data on the influence of the odours of raw plant materials and products on the behaviour of T. castaneum. The present study therefore focused on examining how the preference of T. castaneum for feed materials (wheat bran, coarse wheat meal, corn feed flour) and feed products (compound feed for pigs and for laying hens), both infested and uninfested, affects its behaviour using a preference and an olfactometer test. Additionally, the results revealing the potential of different substrates' odours in attracting T. castaneum are expected to provide information about the susceptibility of different feed substrates to infestation by this pest. Such information has the potential for future improvement of the existing protection programmes for this group of feed materials and products.
Materials and methods
Tested insects
A laboratory population of T. castaneum was reared in 2.5 litres glass jars on soft wheat flour supplemented with 5% dry yeast at the Institute of Pesticides and Environmental Protection, Belgrade, Serbia, according to a procedure described by Harein and Soderstrom (Reference Harein, Soderstrom and Smith1966) and Bry and Davis (Reference Bry, Davis, Singh and Moore1985). In the insectary, the ambient temperature was 25 ± 1°C and relative humidity (RH) 60 ± 5%. Adults used in the experiment were aged 2–4 weeks, unsexed and starved for 24 h before testing.
Feed materials and products
Raw plant materials from the feed industry were used in the tests: coarse wheat meal made from cv. ‘NS 40S’ (Institute of Field and Vegetable Crops, Novi Sad, Serbia), wheat bran (Letina d.o.o, Novi Bečej, Serbia), corn feed flour (Tisa d.o.o, Mirotin, Serbia) and compound feed products for pigs (Letina d.o.o) and for laying hens (Letina d.o.o), with characteristics described by Đukić et al. (Reference Ðukić, Radonjić, Lević, Spasić, Kljajić and Andrić2016). In addition to these raw plant materials and feed products, a standard substrate for rearing T. castaneum in the laboratory consisting of soft wheat flour and 5% yeast was included in the experiments.
The procedure for preparing uninfested experimental substrates was described by Đukić et al. (Reference Ðukić, Radonjić, Lević, Spasić, Kljajić and Andrić2016). The infested substrates were prepared using a modified method described by Trematerra et al. (Reference Trematerra, Ianiro, Athanassiou and Kavallieratos2013), i.e. by providing 100 g of substrate to 100 unsexed T. castaneum adults (1 insect per 1 g of substrate) over a period of 15 days, after which insects were sieved off and the substrates left to rest for the next 24 h in open containers in separate chambers at room temperature before use.
Preference test
The preference test examined the influence of raw plant materials and feed products on the behaviour of T. castaneum adults. Insects used in this experiment were allowed olfactory, visual and contact cues. Plastic arenas, 24 × 18 cm in size, were used, and substrates were offered at diagonal corners. The control area contained no substrate. A single insect per arena was placed in the centre (one insect = one replicate), with a total of 20 insects per test. Each arena was then covered with a bell jar to prevent the insects from escaping, volatiles from evaporating and interference from the environment and other tested units. Insect movement was monitored for 30 min and the position of each was recorded at 3 min intervals (with 10 visits). The arenas were cleaned after each test with 96% alcohol.
We compared the following T. castaneum preferences for:
(a) Uninfested substrates vs. areas with no substrate (six combinations)
(b) Infested substrates vs. areas with no substrate (six combinations)
(c) Uninfested vs. infested substrates (six combinations)
(d) Different uninfested substrates (15 combinations)
(e) Different infested substrates (15 combinations)
The experiment comprised a total of 48 combinations.
Olfactometer test
A two-choice olfactometer (Ninkovic et al., Reference Ninkovic, Dahlin, Vucetic, Petrovic-Obradovic, Glinwood and Webster2013) was used to determine the influence of the odours of infested and uninfested substrates on the behaviour of T. castaneum adults. Both ends of the olfactometer were connected to plastic tubes with containers holding test substrates that had wire-mesh covers to allow airflow over the substrate. The tubes contained filters (cotton wads and active charcoal) to purify the air and prevent the accumulation of flour particles inside the olfactometer device. Airflow was provided by a vacuum pump and circulated over each substrate, carrying its odour into the olfactometer arms and further on into the central zone, which was connected by a tube to the vacuum pump. An empty container without substrate odour was used as the control.
A single insect was placed in the central zone and left to adapt to the new environment for 3 min. Over the next 10 min, its position and movement inside the olfactometer were recorded, i.e. whether it moved into either arm and for how long. The olfactometer was cleaned with 96% alcohol after each test. As with the previous test, this test was conducted in 20 repetitions (one insect = one repetition). Prior to each insect test, the olfactometer was rotated by 180° to avoid positional bias. The same combinations as in the preference test were examined.
Data on the position of insects within plastic containers in the preference test and on the duration of stay in each olfactometer arm were processed by the Wilcoxon test for paired samples (StatSoft, 2011), with a significance level of P ≤ 0.05.
Analysis of protein and starch contents in the substrates
Analysis of the protein and starch contents served to assess the overall chemical composition of uninfested substrates, to compare it to that of infested substrates and to determine if the chemical composition of an uninfested substrate could be one of the reasons for its attractiveness to T. castaneum.
Protein and starch contents were determined in raw plant materials and feed products in the uninfested and infested forms. Uninfested substrates were prepared according to the procedure described by Đukić et al. (Reference Ðukić, Radonjić, Lević, Spasić, Kljajić and Andrić2016). Infested substrates were prepared as five parent pairs that were placed in plastic containers with 10 g of the tested substrates (1 insect per 1 g) in four replicates. The containers were covered with cotton cloth, fixed with rubber bands and put in an incubator (Sutjeska, Serbia) set to 30 ± 1°C and 50 ± 5% RH. Insects were sexed to ensure the production of offspring. The parents were allowed to feed and oviposit for 7 days, after which they were gently removed by sieving and the containers were returned to the incubator. Containers with substrates were kept under the same (described) conditions until eclosion of all offspring, when the substrates were removed for analysis.
Protein and starch contents in uninfested and infested substrate samples were analysed in the laboratories of the Institute of Food Technologies in Novi Sad. Samples for analysis (four replicates) included 10 g of uninfested and infested substrates. The protein content before and after infestation was determined using the Kjeldahl method (Kamizake et al., Reference Kamizake, Goncalves, Zaia and Zaia2003; Junsomboon and Jakmunee, Reference Junsomboon and Jakmunee2008; Finete et al., Reference Finete, Gouvea, Marques and Netto2013) on a Kjeldahl nitrogen determination apparatus (BϋCHI, Labortechnik AG, Switzerland). The starch content before and after infestation was determined using a polarimeter (Pan et al., Reference Pan, Zhu and Cao2007; Yao et al., Reference Yao, Chu, He and Si2014), manufactured by Carl Zeiss, Jena, GmbH, Germany.
One-way ANOVA was used for comparing protein and starch contents in uninfested and infested forms, and the means were separated using the Tukey–Kramer (HSD) test at P = 0.05. The data were processed in StatSoft version 10 (StatSoft Inc., Tulsa, OK, US).
Results
Preference test
Uninfested substrates vs. no-substrate area (control)
Insects participating in the preference test spent significantly more time in the part of the arena containing uninfested substrates than in the no-substrate part. They spent the longest time, 8.15 out of 10 visits on average, in flour supplemented with brewer's yeast and in compound pig feed (7.1 out of 10 visits), while the compound feed for laying hens and coarse wheat meal, where they spent 6 and 6.5 visits, were the least attractive to them (fig. 1a).
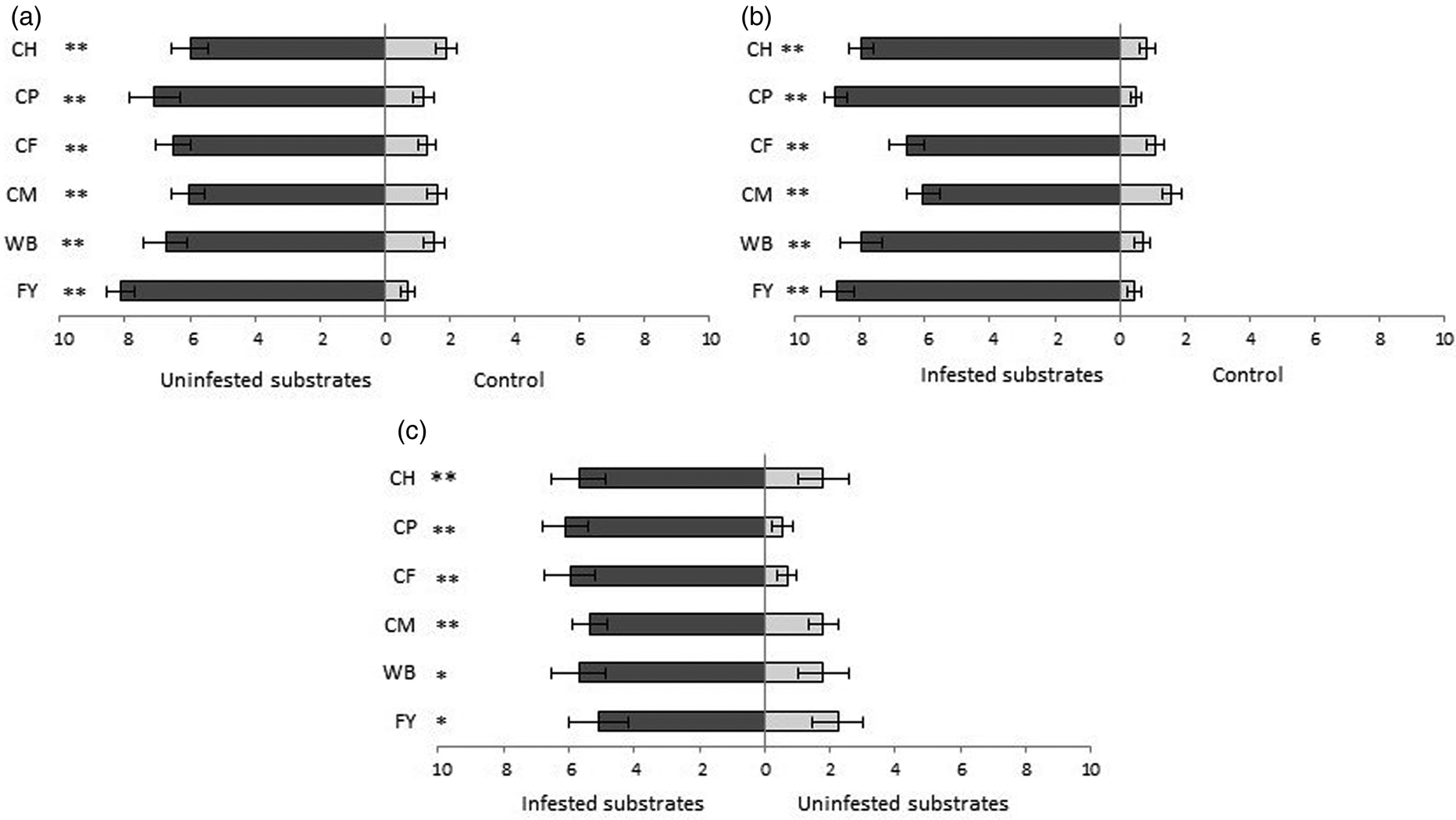
Figure 1. Number of the visits (out of 10 in total) + SE that T. castaneum spent in an arena with: (a) uninfested substrates vs. control (area without substrate), (b) infested substrates vs. control (area without substrate) and (c) infested vs. uninfested substrates. CH, compound feed for laying hens; CP, compound feed for pigs; CF, corn feed flour; CM, coarse wheat meal; WB, wheat bran; FY, flour + yeast. *P < 0.05, **P < 0.01 and NS, no significant difference based on the Wilcoxon pair test.
Infested substrates vs. no-substrate area (control)
Insects were significantly more attracted to infested substrates. Pig feed and flour with yeast were found to be the most attractive. Of the 10 visits, insects mostly chose infested substrates (8.75 and 8.7 visits, respectively). The least attractiveness was that of infested corn feed flour and coarse wheat meal, 6.55 and 7.15, respectively, out of the 10 visits (fig. 1b).
Uninfested vs. infested substrates
Infested substrates were significantly more attractive to insects than uninfested substrates. The greatest differences in the attractiveness of infested substrates when compared to uninfested substrates were found for hen feed (Z = 3.509, P = 0.0004, n = 20), where the ratio was 6.1:0.6, and for pig feed (Z = 2.857, P = 0.004, n = 19), where the ratio was 7.9:1 (fig. 1). The smallest differences between the attractiveness of infested and uninfested substrates (Z = 2.153, P = 0.03, n = 19) were found for flour with yeast, where the ratio was 5.4:1.84, and for coarse wheat meal (Z = 3.101, P = 0.001, n = 17), with a 5.35:1.8 ratio (fig. 1c).
T. castaneum preferences for different uninfested substrates
Wheat bran was the most attractive of all five test combinations. The substrate was significantly more attractive to the insects than flour with yeast and hen feed (Z = 3.92, P = 0.0009, n = 20 and Z = 3.92, P = 0.00009, n = 20, respectively) or coarse wheat meal, pig feed or corn feed flour (Z = 3.621, P = 0.0003, n = 20; Z = 3.4, P = 0.0007, n = 20 and Z = 3.285, P = 0.001, n = 20, respectively) (fig. 2). Flour with yeast was the second most attractive substrate (four of five combinations), followed by corn feed flour (three of five combinations). Coarse wheat meal was the least attractive compared to any other substrate in the five combinations (fig. 2).
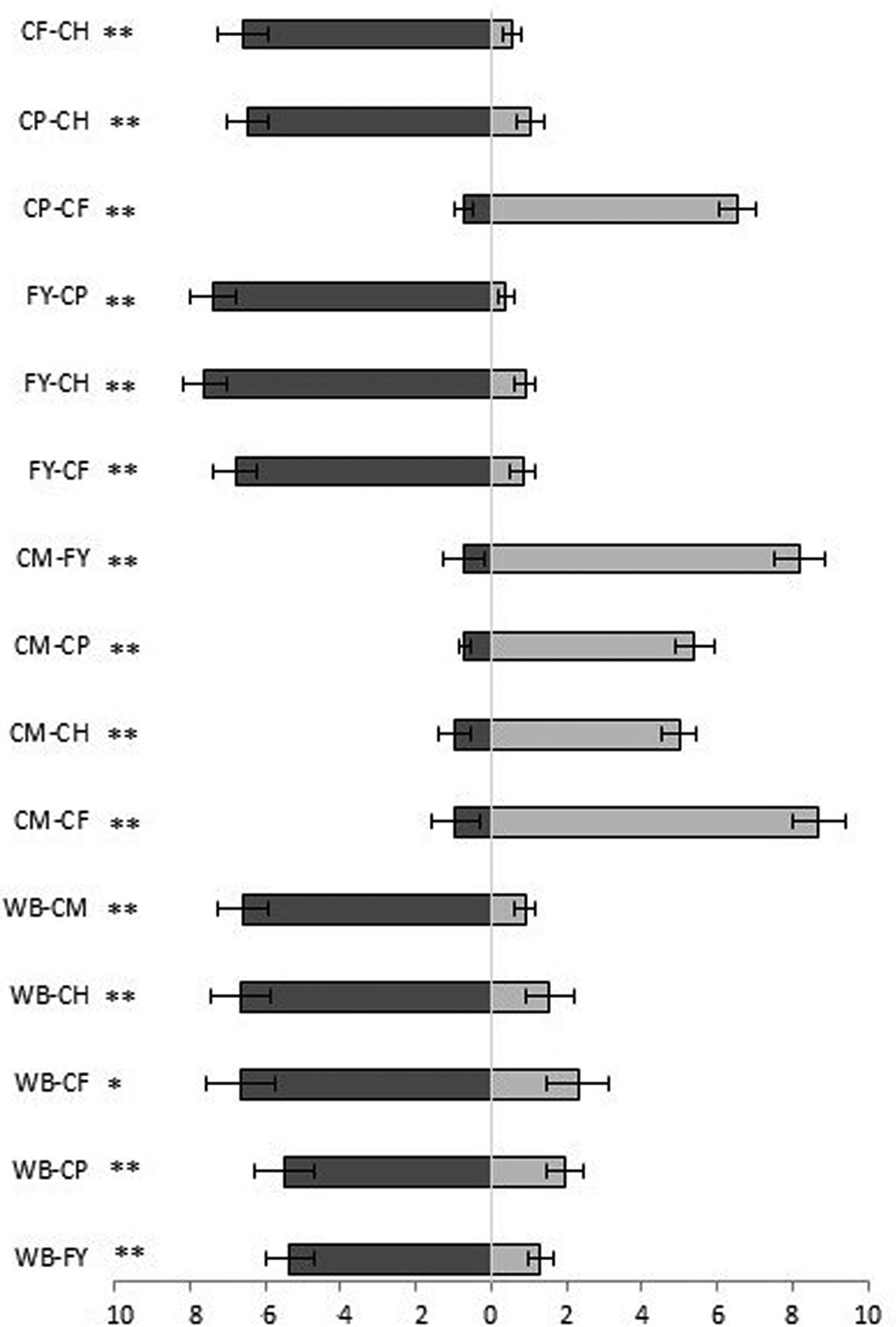
Figure 2. Attractiveness of volatiles of uninfested substrates expressed as the number of the visits (out of 10) + SE that insects spent in either side of the arena. CH, compound feed for laying hens, CP, compound feed for pigs; CF, corn feed flour; CM, coarse wheat meal; WB, wheat bran; FY, flour + yeast. *P < 0.05, **P < 0.01 and NS, no significant difference based on the Wilcoxon pair test.
T. castaneum preferences for different infested substrates
Wheat bran was once more the most attractive of all five combinations in this experiment, attracting insects significantly more than flour with yeast (Z = 2.012, P = 0.04, n = 17), pig feed (Z = 3.043, P = 0.002, n = 20), hen feed (Z = 3.323, P = 0.0009, n = 20), coarse wheat meal (Z = 3.453, P = 0.0005, n = 20) and corn feed flour (Z = 3.561, P = 0.0004, n = 19) (fig. 3). Coarse wheat meal was the least attractive to insects, but the attractiveness of pig feed and hen feed was also not significantly greater than the other substrates (fig. 3).
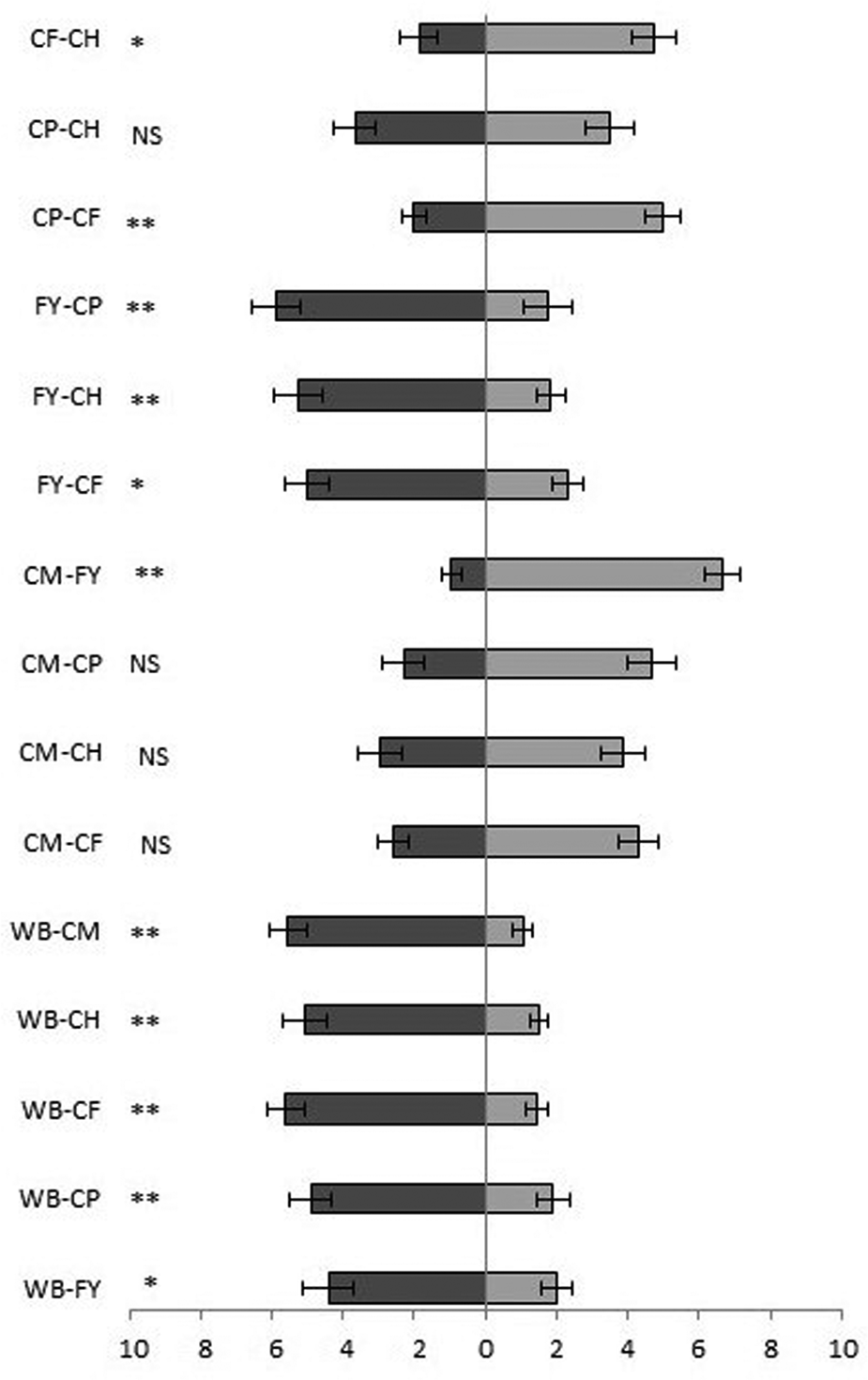
Figure 3. Attractiveness of volatiles of infested substrates expressed as the number of the visits (out of 10) + SE that insects spent in either side of the arena. CH, compound feed for laying hens; CP, compound feed for pigs; CF, corn feed flour; CM, coarse wheat meal; WB, wheat bran; FY, flour + yeast. *P < 0.05, **P < 0.01 and NS, no significant difference based on the Wilcoxon pair test.
Olfactometer bioassay
Uninfested substrates vs. air without substrate odour (control)
The odours of all uninfested substrates were significantly more attractive to insects than the odourless control (fig. 4a). Insects were most attracted by the odours of uninfested flour with yeast and wheat bran; they spent 536.25 out of the 600 s in the arm with flour odours and 521.25 s in the olfactometer arm with bran odour, while they spent less time in the arm with hen feed (423.75 s) and coarse wheat meal (470.5 s) odours.
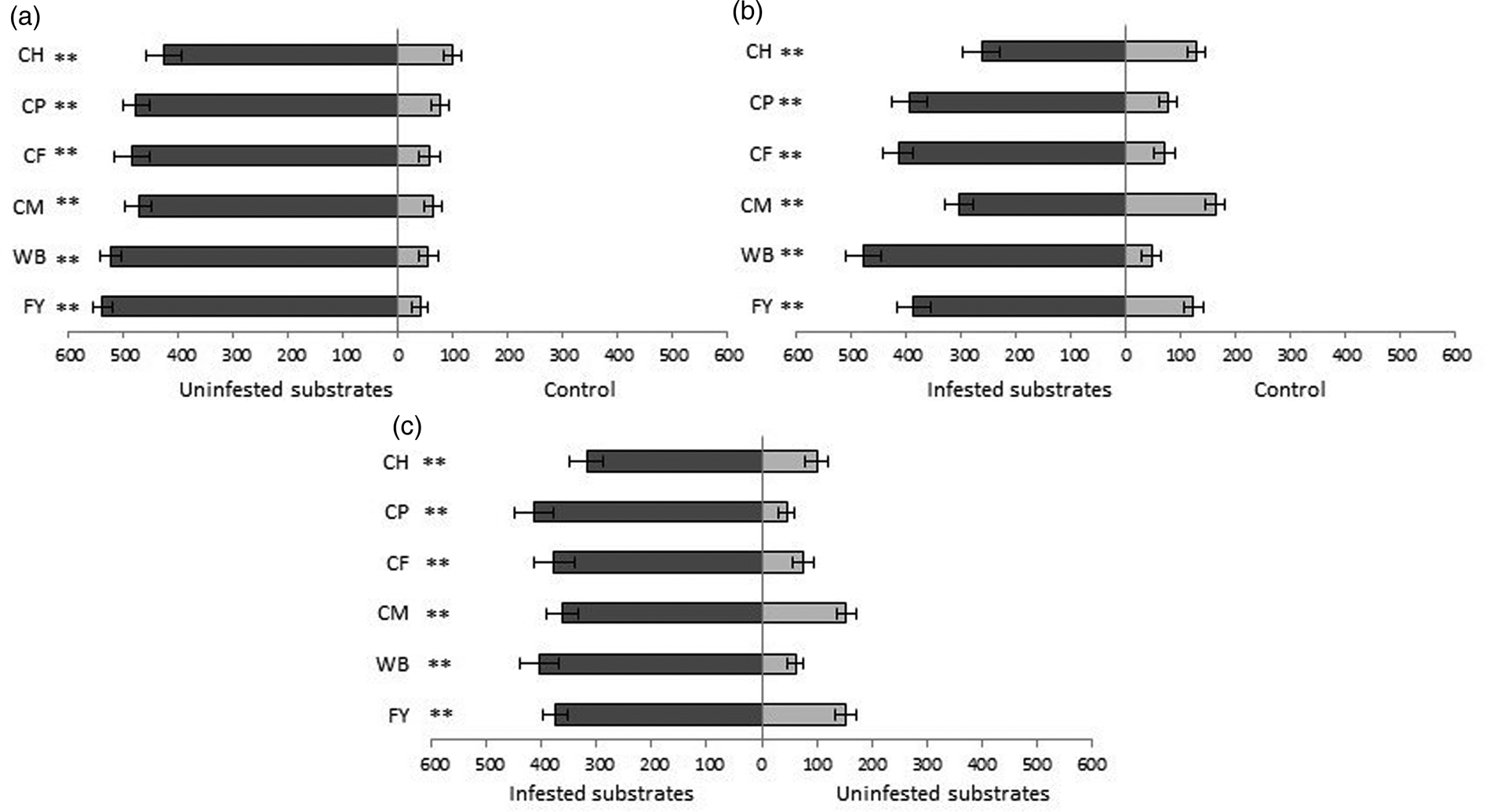
Figure 4. Time (seconds) + SE that T. castaneum spent in each arena of the two-arm olfactometer; (a) uninfested substrates vs. control (area without substrate), (b) infested substrates vs. control (area without substrate) and (c) infested vs. uninfested substrates. CH, compound feed for laying hens, CP, compound feed for pigs; CF, corn feed flour; CM, coarse wheat meal; WB, wheat bran; FY, flour + yeast. *P < 0.05, **P < 0.01 and NS, no significant difference based on the Wilcoxon pair test.
Infested substrates vs. air without substrate odour (control)
Insects spent significantly more time in the olfactometer arm with the odours of all infested substrates than in the control arm, free of any tested substrate odour (fig. 4b). Of all infested substrates, the odour of wheat bran attracted insects the most, so that they remained in this arm 475.25 out of 600 s; insects spent the least time in the arms with odours of hen feed (261.25 s) and coarse wheat meal (302.25 s).
Uninfested vs. infested substrates
The odour of infested substrates was significantly more attractive to insects than the odour of uninfested substrates (fig. 4c). The greatest differences in the attractiveness of infested compared to uninfested substrates were recorded for wheat bran and pig feed (Z = 3.883, P = 0.0001, n = 20; Z = 3.883, P = 0.0001, n = 20) as insects spent 404.25 and 414 s out of the 600 s in the olfactometer arms with the odours of infested substrates, and only 61.25 and 45.75 s in the arms with odours of the same uninfested substrates (fig. 4c), respectively. The smallest differences were revealed for flour with yeast and coarse wheat meal as insects spent 375.25 and 361.25 s in these olfactometer arms, and in the arms with the same uninfested substrates, they spent 152.25 and 154.25 s (Z = 3.770, P = 0.0002, n = 20; Z = 3.435 P = 0.0006, n = 20), respectively (fig. 4c).
T. castaneum preferences for different uninfested substrate odours
Testing the combinations of all the uninfested substrates, the odour of wheat bran was found to be the most attractive to T. castaneum adults. Bran odour was significantly more attractive than flour with yeast (Z = 3.912, P = 0.00008, n = 20), hen feed (Z = 3.912, P = 0.00009, n = 20), coarse wheat meal (Z = 3.621, P = 0.0003, n = 20), pig feed (Z = 3.397, P = 0.0007, n = 20) and corn feed flour (Z = 3.285, P = 0.001, n = 20) (fig. 5). Slightly lower attractiveness was demonstrated by flour with yeast, which was more attractive than the other substrates in all combinations except with wheat bran (fig. 5). Following in attractiveness were corn feed flour and pig feed, which were significantly more attractive in two of five combinations. Significantly, the least attractiveness was shown by wheat coarse meal, which was less attractive in all five combinations (fig. 5).
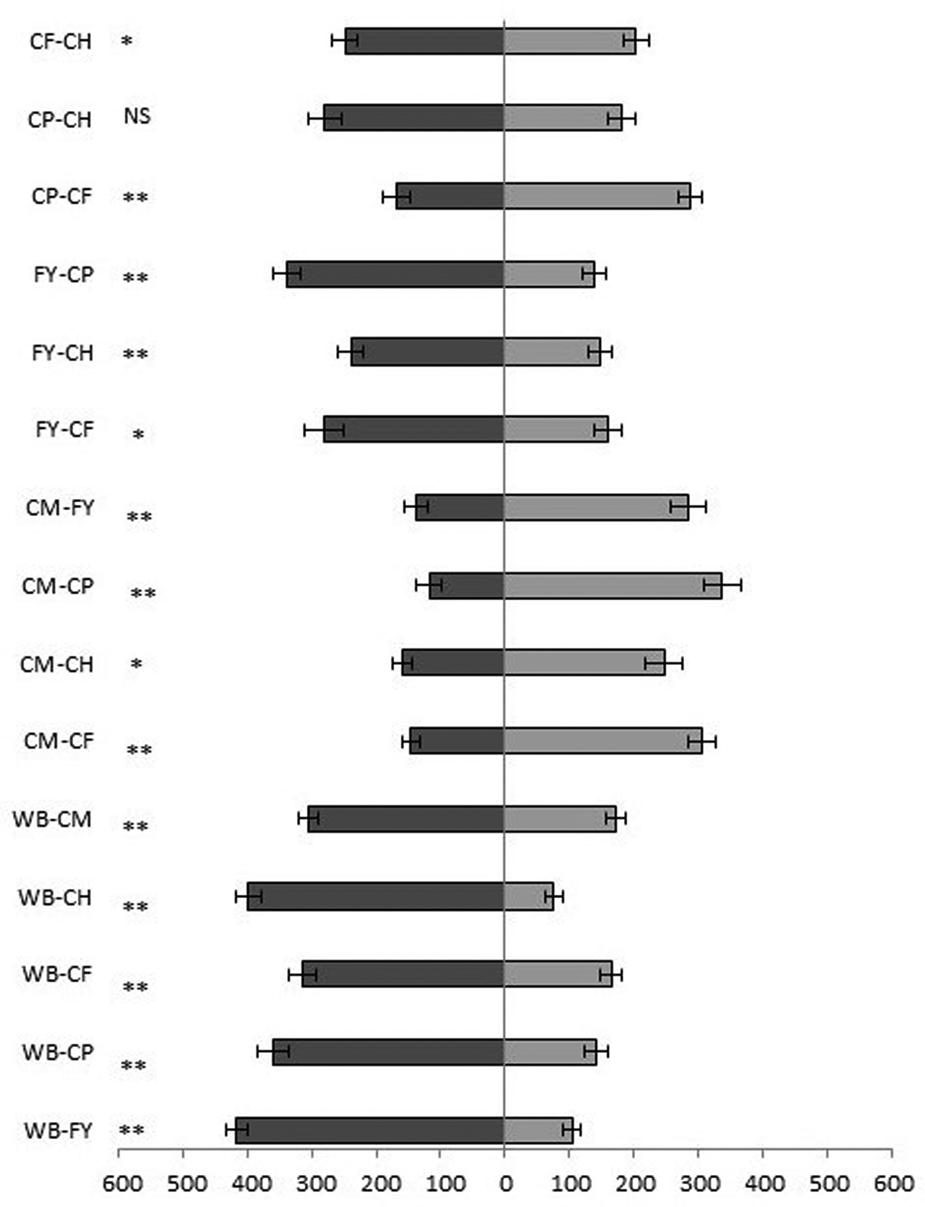
Figure 5. Attractiveness of volatiles of uninfested substrates expressed as the time (seconds) + SE that T. castaneum spent in one or the other olfactory arm. CH, compound feed for laying hens; CP, compound feed for pigs; CF, corn feed flour; CM, coarse wheat meal; WB, wheat bran; FY, flour + yeast. *P < 0.05, **P < 0.01 and NS, no significant difference based on the Wilcoxon pair test.
T. castaneum preferences for different infested substrate odours
Comparing the combinations of all infested substrates, wheat bran demonstrated significantly the greatest attractiveness for T. castaneum adults in all five combinations with other substrates (fig. 6). Wheat bran was followed in attractiveness by flour with yeast, which was significantly more attractive than the other substrates in three of the five combinations. Compared to wheat bran, flour with yeast was significantly less attractive (Z = 3.248, P = 0.001, n = 20), and similar in attractiveness to hen feed (Z = 1.755, P = 0.08, n = 20). Coarse wheat meal was significantly the least attractive in all five combinations compared to the other substrates.

Figure 6. Attractiveness of volatiles of infested substrates expressed as the time (seconds) + SE that T. castaneum spent in an olfactory arm. CH, compound feed for laying hens; CP, compound feed for pigs; CF, corn feed flour; CM, coarse wheat meal; WB, wheat bran; FY, flour + yeast. *P < 0.05, **P < 0.01 and NS, no significant difference based on the Wilcoxon pair test.
Protein and starch contents in uninfested and infested substrates (after offspring development)
Generally, the average protein content was statistically significantly lower (P < 0.05) for all tested infested substrates (after offspring development) compared to the percentage of protein in the same uninfested substrates (table 1). In different substrates, the decrease in protein content was differently expressed. Thus, the greatest reduction in infested substrate protein was observed in bran, which was 16.4% before development and 12.93% after offspring development (table 1). The average starch content was also statistically significantly lower (P < 0.05) in the test infested substrates. Like proteins, the greatest decrease in starch was observed in infested bran (the percentage of starch before the development of offspring was 25.93% and after development only 16.11%).
Table 1. Average protein and starch content (% ± SE) of substrates before and after insect development
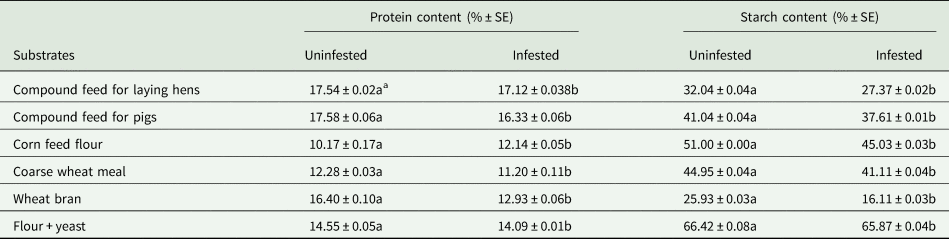
a Each mean is based on four replications. For protein content and starch content separately, means within rows followed by the same letter are not significantly different, Tukey–Kramer (HSD) test at P = 0.05.
Discussion
The results of the present study revealed the great influence of the odours of feed materials and feed products on the searching behaviour of T. castaneum adults. The odours of uninfested substrates showed a significant attractiveness to the species, while infestation of substrates additionally enhanced their attractiveness. The most attractive substrate was wheat bran in both uninfested and infested forms. Coarse meal, even though it is a product of wheat grain, was the least attractive. The preference test showed substrate attractiveness but it allowed the involvement of all cues (olfactory, visual and contact), while the olfactometer allowed insects to use only olfaction and it confirmed that T. castaneum adults primarily rely on the sense of smell in their search for feed.
The high substrate attractiveness observed in this experiment may be attributed to the fact that all test substrates were mechanically damaged (ground) and that T. castaneum, as a secondary pest, feeds on damaged grain. Earlier studies showed that T. castaneum adults were significantly more attracted by the odours of grain parts, ground wheat and millet grain than they were by the odour of whole grains of the same crops (Seifelnasr et al., Reference Seifelnasr, Hopkins and Mills1982; Phillips, Reference Phillips1997; Trematerra et al., Reference Trematerra, Sciarreta and Tamasi2000; Ahmad et al., Reference Ahmad, Daglish, Ridley and Walter2012). Contrary to these previous and our present results, Stevenson et al. (Reference Stevenson, Cai, Faucher, Michie, Berna, Ren, Anderson, Chyb and Xu2017) did not confirm the attractiveness of either whole or ground grains of wheat to T. castaneum adults, but found larvae to be attracted, without showing a preference for grain or flour odours. In the mentioned studies, and summing up both tests, all substrates were significantly attractive to insects, and uninfested substrates were 3–13 times more attractive than areas without substrate.
Secondary pests generally colonize substrates that have already been damaged by primary pest feeding; they are more strongly attracted by the odours of substrates damaged by primary pests than by mechanical means (Trematerra et al., Reference Trematerra, Sciarreta and Tamasi2000, Reference Trematerra, Ianiro, Athanassiou and Kavallieratos2015). As the focus so far has been on the interaction between primary and secondary pests, the reaction of T. castaneum and other secondary pests to the odours of food infested by their own species has not been adequately researched. A recent study has confirmed the attractiveness of flour containing T. castaneum adults to the larvae and other adults of the same species (Stevenson et al., Reference Stevenson, Cai, Faucher, Michie, Berna, Ren, Anderson, Chyb and Xu2017). The present study used substrates infested with the same species which were then left to rest for 24 h without any insect presence. Such substrates were significantly more attractive than the no-substrate control, so that the infested substrates were 6–19 times more attractive than the area without substrates in the preference test, and 2–10 times more attractive than the clean-air arm in the olfactometer test. In choosing between uninfested and infested substrates, insects were significantly more attracted to infested substrates. Depending on the substrate, the infested substrates in both tests attracted insects 2–11 times more than uninfested substrates. The results indicating a high attractiveness of substrates that had been infested previously with T. castaneum offer insight into the intraspecific behaviour of this species. This high attractiveness of infested substrates in our experiment may be attributed to semiochemicals secreted by the insects, but also to an interaction between substrate and semiochemicals, i.e. to the volatiles created by insect secretion during feeding and mixing with the odour of substrates. This observation is confirmed by the fact that uninfested substrates that were less attractive to insects than other substrates also had weaker attractiveness after infestation. This poses new questions requiring further chemical analysis of the volatiles of infested substrates.
Insects demonstrated clear preferences for different tested feed materials and products. Comparing the attractiveness of each uninfested and infested substrate, wheat bran was found to be the most attractive to T. castaneum in both tests as it attracted the insects significantly more than any other uninfested or infested substrate. Seifelnasr et al. (Reference Seifelnasr, Hopkins and Mills1982) also reported the high attractiveness of wheat bran volatiles, which they found to be more attractive to T. castaneum than those of undamaged wheat grain. Wheat bran was the substrate in which T. castaneum produced the largest number of progeny with the shortest cycle of development compared to other substrates tested in our earlier experiment (Đukić et al., Reference Ðukić, Radonjić, Lević, Spasić, Kljajić and Andrić2016). The ability of stored-product insects to choose the most suitable substrate for feeding and reproduction based on their sense of smell has been noted in some earlier studies showing that T. castaneum and the lesser grain borer Rhyzopertha dominica (F.) were significantly more attracted by the odours of substrates in which the species have the highest reproduction and fastest offspring development than by the odours of those in which their progeny is small, develops more slowly and has a smaller body mass (Bekon and Lassard, Reference Bekon and Lassard1988; Edde and Phillips, Reference Edde and Phillips2006). Flour with yeast and pig feed were the next most attractive diets after wheat bran in the olfactometer test. Pig feed is a complex of several components, one of which is yeast, which can increase attractiveness, as confirmed earlier (Bergerson and Wool, Reference Bergerson and Wool1987). It is noteworthy that bran and coarse wheat meal have the same nutritive components, and yet one was the most and the other the least attractive to T. castaneum adults. We assume that coarse meal, i.e. roughly fragmented wheat grain, is unable to exude the same amount of volatiles as bran, whose flattened structure and large area gives off more volatiles that are attractive to insects. Also, the structure of coarse meal is less suitable for feeding, reproduction and development because T. castaneum prefers finer structures (Li and Arbogast, Reference Li and Arbogast1991; Fardisi et al., Reference Fardisi, Mason and Ileleji2013; Đukić et al., Reference Ðukić, Radonjić, Lević, Spasić, Kljajić and Andrić2016).
The literature data show that the attractiveness of uninfested substrates to secondary pests depends a great deal on mechanical damage, particle shape and size (Freedman et al., Reference Freedman, Mikolajczak, Smith, Kwolek and Burkholder1982; Bergerson and Wool, Reference Bergerson and Wool1987; Trematerra et al., Reference Trematerra, Sciarreta and Tamasi2000; Campbell, Reference Campbell2012; Trematerra et al., Reference Trematerra, Ianiro, Athanassiou and Kavallieratos2015). There are no available data on the chemical composition of substrates and its role in attractiveness to T. castaneum. We wanted to examine if there was another reason for the different attractiveness of the substrates. We observed the qualitative structure of the substrates for which we had data (manufacturers' classifications) (Đukić et al., Reference Ðukić, Radonjić, Lević, Spasić, Kljajić and Andrić2016). As the content of carbohydrates was dominant in all the tested substrates, and carbohydrates are a broad group of chemical compounds, we decided to do an additional quantitative analysis of starch and protein. Analysis showed that the smallest percentage difference between protein and starch was found in bran. To determine whether this relationship was relevant for offspring development, we performed a chemical analysis of the substrates after tdevelopment of the species' offspring. Comparing the amounts of starch and protein before and after offspring development, we found that the highest amounts of carbohydrates and proteins relative to the initial values were consumed in wheat bran. This tells us that a balanced diet is appropriate for this species, as confirmed by previous studies (Đukić et al., Reference Ðukić, Radonjić, Lević, Spasić, Kljajić and Andrić2016). We assume that the attractiveness of a substrate, besides its physical structure, also depends on its chemical structure and this is directly related to optimal development conditions, i.e. the possibility of choosing the most suitable place for producing the most offspring, which is a basic biological role of all living organisms.
In conclusion, the present study showed the impact of feed materials and products on the behaviour of T. castaneum, and examined further the insufficiently clear and complex host–insect relationship in feed stores. A significantly greater attractiveness of infested plant material compared to uninfested was confirmed, which contributes to a better understanding of the intraspecific behaviour of this species, while insect preference for certain substrates gives a clearer idea of their susceptibility to infestation by this insect pest. The high attractiveness of wheat bran to T. castaneum makes this type of feed the most prone to infestation, so that any further research should include other stored-product insect pests to determine their overall potential as attractants.
Acknowledgement
This study was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Projects: III 46008 and III 46012). Our thanks to the Laboratory of the Institute of Food Technology (FINS) in Novi Sad, Serbia, for chemical analysis of the protein and starch contents in substrates.












