Wholegrain consumption has had a recognised negative correlation with risk of CVD( Reference Amano, Kawakubo and Lee 1 ), diabetes( Reference Liu, Manson and Stampfer 2 ), obesity( Reference van de Vijver, van den Bosch and van den Brandt 3 ) and certain cancers( Reference Chan, Wang and Holly 4 ). From the early 1940 s, the US Department of Agriculture recommended the daily intake of wholegrains and, in 2008, the Food and Drug Administration allowed a health claim that eating wholegrain reduces the risk of heart disease( 5 ). It has been proposed that wholegrains contain a combination of bioactive components, for example, dietary fibre, phytochemicals and vitamins, that all have individual roles to play, to provide widespread protection from cardiometabolic diseases( Reference Jacobs and Gallaher 6 ). Wholegrains have a multitude of compounds; some of these components have been individually investigated and evaluated for their role in the protective mechanisms against chronic disease risk.
The glycaemic index (GI) is a functional test that describes the quality of a carbohydrate. It is defined as the blood glucose-raising potential of a standard quantity of carbohydrate, compared with a glucose control. After consumption of high-GI carbohydrates, blood glucose rapidly increases. This stimulates the release of insulin from β-cells, required for the transport and metabolism of glucose. The extremes in circulating glucose and insulin concentrations are associated with eventual pancreatic β-cell exhaustion and increased metabolic stress( Reference DeFronzo 7 ). Epidemiological studies have reported association between the consumption of low-GI foods and reduced risk of CVD, diabetes and certain cancers( Reference Du, van der and van Bakel 8 – Reference Giovannucci 11 ). One component of foods which is associated with a lower GI is the presence of intact wholegrains( Reference Atkinson, Foster-Powell and Brand-Miller 12 ). More recently, interest has focused on the potential impact of wholegrain cereals on the intestinal microbiota and the possible contribution of this mechanism to the observed reduced risk of cardiometabolic disease( Reference Costabile, Klinder and Fava 13 , Reference Carvalho-Wells, Helmolz and Nodet 14 ). An imbalance of the intestinal microbiota is thought to predispose individuals to a variety of diseases and disorders, for example, inflammatory bowel disease and obesity( Reference Macfarlane, Blackett and Nakayama 15 ). The term prebiotic describes non-digestible soluble fibres such as inulin, oligosaccharides and resistant starch (RS), which are selectively fermented by groups of beneficial bacteria present in the gut. The diet–microbiota association (in particular Bifidobacterium and Lactobacillus genera) is now thought to have an important role in human health( Reference Costabile, Klinder and Fava 13 ). One of the main by-products of gut fermentation are SCFA. Butyrate has a major role in colonocyte proliferation and differentiation, and an increased concentration has been associated with reduced colon cancer incidence( Reference Roediger 16 – Reference Bornet, Brouns and Tashiro 18 ). In human feeding studies often no effect is observed, as over 90 % of SCFA are absorbed in the colon and utilised by the host. It is therefore important to establish the quantity and ratio of SCFA produced on fermentation of the non-digestible components of wholegrain cereals through in vitro methods.
There are four mechanisms that have been proposed to have roles in the protectiveness of wholegrains. They are: effect on the large bowel; effect on blood glucose and insulin levels; antioxidants; and, lastly, other bioactive compounds that may differ between different wholegrains. As stated previously, most of these mechanisms have been investigated using individual components of wholegrains. Components such as dietary fibre, vitamins, minerals, lignans and phytochemicals are found in the bran part of grain or just below this layer. On refining wholegrain, the bran is removed and as a result these bioactive compounds are lost. It can be concluded that wholegrain cereal consumption at breakfast time can be associated with maintaining a healthy lifestyle and this may play a role in protection from metabolic disorders. Processing of wholegrains has previously been shown to have a negative impact on glycaemic response and to have an impact on the gut fermentation profile( Reference Connolly, Lovegrove and Tuohy 19 ). In the present study we used pH-controlled, faecal batch cultures as an in vitro mixed culture system to investigate the microbiota-modulating capabilities of five different wholegrain cereals, which have been processed to differing extents, towards a more beneficial composition. Additionally we determined the GI of these different cereals in human volunteers. This is a novel investigation, which may have implications for designing the optimal breakfast cereals aimed at maintaining health-promoting activities of wholegrains.
Material and methods
Substrates
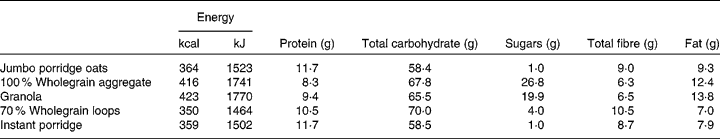
Two control samples, cellulose (Oxoid), which was not expected to be associated with bacterial growth, and oligofructose (Raftilose P95; Beneo-Orafti), a confirmed prebiotic, were included in the in vitro fermentation experiment. The breakfast cereals tested were: jumbo porridge oats (Traditional Porridge®; The Jordans and Ryvita Company Ltd), 100 % wholegrain aggregate (Original Crunch®; The Jordans and Ryvita Company Ltd), granola (Superfoods Granola®; The Jordans and Ryvita Company Ltd), 70 % wholegrain loops (Cheerios®; General Mills, Inc.) and instant porridge (Readybrek®; Weetabix Food Company). The nutritional information of these cereals is listed in Table 1.
Table 1 Nutritional information of breakfast cereals investigated (per 100 g cereal)
Simulated human digestion of food (from mouth to small intestine)
Cereal sample (240 g) was weighed into a stomacher bag (Seward classic 400), sterile distilled water (300 ml) was added and the mix blended for 5 min at normal speed using a stomacher (Seward stomacher 400). α-Amylase (Sigma) was dissolved in filtered sterilised CaCl2 (1 mm; pH 7·0); this solution and the blended cereals were dispensed into a 500 ml glass Duran® bottle (Sigma). The mix was incubated at 37°C for 30 min on a shaker (IKA labortechnik KS501) set at 120 g . The pH was acidified to pH 2 with HCl (6 m), pepsin (Sigma) was dissolved in HCl (0·1 m) at a ratio of 1:15 (w:v) and added to the mix and incubated for a further 2 h at 37°C, shaking. The pH of the mix was adjusted to 6·5 using NaOH (6 m). Pancreatin (Sigma) and bile extract (Sigma) were dissolved with filtered sterilised NaHCO3 (0·5 m) and dispensed into the mix. The pH was adjusted to pH 7·0 using either a strong acid or base and incubated for 3 h at 37°C, shaking. Cold, sterile NaCl solution (10 mm) was added and the mix was poured into dialysis tubing (Spectra/Por 1 kDa molecular-weight cut-off (MWCO) dialysis membrane; Spectrum) and incubated overnight at 5°C. The following morning the dialysis tubing was replaced with fresh NaCl solution and incubated for a further 2 h. The dialysis tubes were then removed and held over a clean sterile pot. The cereal mix was collected and dispensed into several 250 ml clear plastic, metal cap containers (Barloworld Scientific) and stored at − 80°C overnight. The frozen samples were then freeze-dried (IEC lyoprep 3000) for 1 week to remove all fluid content.
Faecal batch culture fermentation
The fermentation profile of digested freeze-dried cereals, the prebiotic oligofructose (Raftilose P95; Beneo-Orafti) and the lowly fermented control non-prebiotic carbohydrate cellulose (Oxoid) was determined using anaerobic, pH-controlled faecal batch cultures. Sterile stirred batch culture fermentation vessels (200 ml working volume) were prepared and aseptically filled with 180 ml of sterile basal nutrient medium. Basal medium contained (per litre): 2 g peptone, 2 g yeast extract, 0·1 g NaCl, 0·04 g K2HPO4, 0·04 g KH2PO4, 0·01 g MgSO4·7H2O, 0·01 g CaCl2·6H2O, 2 g NaHCO3, 2 ml Tween 80, 0·05 g Hemin dissolved in 1 ml of 4 m-NaOH, 10 μl vitamin K (Sigma), 0·5 g l-cysteine HCl, and 0·5 g bile salts (sodium glycocholate and sodium taurocholate). The medium was adjusted to pH 7·0 and 4 ml of 0·025 % (w/v) resazurin solution added before autoclaving. Once in the fermentation vessels, the sterile medium was sparged with O2-free N2 (15 ml/min) overnight to maintain anaerobic conditions. The following day test substrates were allowed to dissolve in the basal medium to give a final concentration of 1 % (w/v) before inoculation of the vessels with 10 % (w/v) faecal slurry, which was prepared with pre-reduced sterile PBS (pH 7·0). Faecal samples were collected from three healthy faecal donors. All donors were healthy males aged between 23 and 31 years, and had not received antibiotic treatment for at least 3 months before stool collection, had not consumed pre- or probiotic supplements immediately before experimentation, and had no history of bowel disorders. The temperature of the fermentation vessels was held at 37°C by use of a circulating water-bath, pH values were held between 6·7 and 6·8 by the addition of 0·5 m-NaOH or -HCl to the vessels, pH was controlled via pH meter controllers (Electrolab260; Electrolab Ltd) and anaerobic conditions were maintained by sparging the vessels with O2-free N2 (15 ml/min). A 5 ml sample was taken from each vessel immediately for analysis; similarly, samples were taken at 5, 10 and 24 h for analysis of SCFA by GC and for analysis of bacterial populations by fluorescence in situ hybridisation (FISH). This experiment was performed in triplicate.
Culture-independent enumeration of faecal bacteria using 16S rRNA probes labelled with Cy3 and fluorescence in situ hybridisation
Changes in faecal bacteria populations upon fermentation of the test substrates were monitored using FISH. FISH was performed essentially as described by Daims et al. ( Reference Daims, Stoecker and Wagner 20 ). Briefly, faecal batch culture samples (375 μl) were fixed using ice-cold 4 % paraformaldehyde (pH 7·2) at a ratio of 1:3 (v/v) for 4 h at 4°C. They were then centrifuged at 13 000 g for 5 min and washed twice in 1 ml filtered PBS. The remaining pellet was re-suspended in a filtered sterilised PBS–ethanol mix (1:1, v/v) and stored at − 20°C for up to 3 months.
For the hybridisations, 20 μl of each sample were pipetted onto Teflon- and poly-l-lysine-coated, six-well (10 mm diameter each) slides (Tekdon, Inc.). Samples were dried onto the slides at 46°C for 15 min and afterwards dehydrated in an alcohol series (50, 80 and 96 %, 3 min each). The ethanol was allowed to evaporate from the slides before the probes were applied to the samples. To permeabilise the cells for use with the probe Lab158, samples were treated with 50 μl of lysozyme (1 mg/ml in 100 mm-2-amino-2-hydroxymethyl-propane-1,3-diol (Tris)-HCl; pH 8·0) at 37°C for 15 min before being washed briefly (2–3 s) in water and afterwards dehydrated in the ethanol series. A probe–hybridisation buffer mixture (5 μl of a 50 ng/μl stock of probe plus 45 μl of hybridisation buffer) was applied to the surface of each well. Hybridisations were performed for 4 h in an ISO20 oven (Grant Boekel). Slides were stored in the dark at 4°C (for a maximum of 3 d) until cells were counted using a Nikon E400 Eclipse microscope fitted with an epifluorescence attachment. A total of fifteen randomised views were counted for each sample.
The hybridisation was carried out as previously described by Rycroft et al. ( Reference Rycroft, Jones and Gibson 21 ) using genus- and group-specific 16S rRNA gene-targeted oligonucleotide probes labelled with Cy3 (Sigma-Aldrich) or the nucleic acid stain 4′,6-diamidino-2-phenylindole (DAPI) for total cell counts. Oligonucleotide probes used were: Bif164, specific for the Bifidobacterium genus( Reference Langendijk, Schut and Jansen 22 ); Lab158, for the Lactobacillus–Enterococcus group( Reference Harmsen, Elferrich and Schut 23 ); Bac303, specific for the Bacteroides and Prevotella group( Reference Manz, Amann and Ludwig 24 ); His150, for the Clostridium histolyticum subgroup( Reference Franks, Harmsen and Raangs 25 ); Erec482, for the Ruminococcus–Eubacterium–Clostridium (REC) cluster; and Ato291, for the Atopobium cluster, including most Coriobacteriaceae species( Reference Harmsen, Wildeboer-Veloo and Grijpstra 26 ).
Quantification of SCFA by GC
GC was performed to determine changes in SCFA concentration during the fermentation of the cereal digests; the method was adapted from Zhao et al. ( Reference Zhao, Nyman and Jönsson 27 ). A quantity of 1 ml of batch culture was centrifuged at 13 000 g for 10 min and the supernatant fraction removed to a fresh Eppendorf tube and then stored at − 20°C for up to 3 months. An internal standard of ethylbutyric acid was prepared with HPLC gradient water to give a final concentration of 10 mm. Preparation of the external standard containing the SCFA acetic, propionic, butyric, isobutyric, valeric, isovaleric and caproic acids were added to give a final concentration of 25 mm to HCl (6 m) and HPLC gradient water. Dilutions of the external standards were prepared and added to the internal standard (ratio 4:1) to give a final concentration for the internal standard of 2 mm-ethyl butyric, and a final concentration of external standards of 20 mm, 10 mm, 5 mm, 1 mm and 0·5 mm. Samples were defrosted and HCl (6 m) added (ratio 1:3) and left at room temperature for 10 min. Samples were centrifuged at 13 000 g for 5 min, and the supernatant fraction was passed through a 0·2 μm polyvinylidene fluoride (PVDF) filter (Whatman). Internal standard was added to the filtered supernatant fraction (ratio 1:4). Samples were run through a 5890 Series II GC system (Hewlett Packard) fitted with a free fatty acid phase (FFAP) column (30 m × 0·53 mm, diameter 0·50 μm; J&W Scientific) and a flame-ionisation detector. Samples were automatically injected into a fused-silica capillary column. Carrier gas, He, was delivered at a flow rate of 14 ml/min and the split ratio was 10:1. The total flow rate was 140 ml/min. Initial oven temperature was 100°C, maintained for 0·5 min, raised to 150°C at 8°C/min, then increased to 250°C at 50°C/min, and held at 250°C for 2 min. Injector and detector temperatures were set at 280 and at 300°C, respectively. The injected volume of the sample and standards was 1 μl. The SCFA peaks were integrated using Atlas Lab managing software (Thermo Lab Systems) and concentrations were calculated by comparing their peak areas with those of the standards and are expressed as mmol/g faeces.
Measuring glycaemic index of test cereals
A total of twelve volunteers who were non-smokers were recruited to the study. They were five women and seven men, aged 27 (range 23–31) years, with BMI of 23·3 (range 19·0–27·6) kg/m2. At baseline, all subjects had fasting plasma glucose concentrations < 6·1 mmol/l. Exclusion criteria were: an active gastrointestinal or metabolic disease (for example, coeliac disease), first-degree family history of diabetes, and any medication. Pregnant and lactating women were also excluded. Written informed consent was obtained from all subjects before the study. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Reading's Research Ethics Committee. Written informed consent was obtained from all subjects. The participants were served each test food once and both reference foods (50 g and 25 g glucose solution) twice at 1-week intervals in a random order. The size of the meal was designed to provide a portion of cereal that would normally be consumed (either containing 50 or 25 g available carbohydrate). The subjects were asked to avoid vigorous physical activity and alcohol consumption the day before the study days. They were asked to fast for 12 h after their evening meal, to avoid exercise on the morning of the study, and to arrive at the Hugh Sinclair Unit of Human Nutrition by car or public transportation. A baseline finger-prick capillary blood sample was taken, after which the subject consumed the study meal within 10 min. Finger-prick capillary blood samples were obtained at 15, 30, 45, 60, 90 and 120 min after the start of the meal. Jumbo porridge oats and instant porridge were prepared according to package instructions using 125 ml skimmed milk. The other cereals were served with 125 ml skimmed milk. All subjects consumed 150 ml water with the test meal. A 50 and 25 g glucose solution was prepared (dissolved in 150 ml water) and used as a reference and each was tested on two occasions. The quantity of test foods was equivalent to 50 g for all cereals except jumbo porridge oats and instant porridge when 25 g of available carbohydrate was given. A smaller amount of test food was used, as the size of the portion that contained 50 g of available carbohydrate was too large for the participants to consume within the allotted time. Capillary blood glucose was measured directly by using a glucose meter (HemoCue Glucose 201; HemoCue Ltd) that applied a modified glucose dehydrogenase method. A series of quality-control solutions recommended by HemoCue was measured every study morning; the CV of these measurements was 1·1 %. The incremental area under the glucose response curve (IAUC) was determined for each cereal using the fasting level as the baseline and ignoring any area below the baseline. The GI was defined as the IAUC of the blood glucose response curve of a 50 g (or 25 g) available carbohydrate portion of a test meal expressed as a percentage of the response to the same amount of available carbohydrate from the reference food. The reference value and each subject's individual GI for each cereal were calculated. The GI for each cereal was taken as the mean of all twelve individual values.
Statistical analysis
Statistical analysis was performed using Minitab for Windows, version 15. Univariate ANOVA and the post hoc Tukey test were used to determine the significance of the effect of substrates on bacterial group populations and SCFA production. Normality testing was carried out to determine if data were normally distributed. Significant differences for changes in bacterial populations over 24 h between treatments were also determined. Differences were deemed significant when P < 0·05. The IAUC was calculated by the trapezoid rule.
Results
Growth of human colonic microbiota on digested substrates
Changes in bacterial populations after 0, 5, 10 and 24 h fermentation with the different test substrates are shown in Table 2. No significant changes in total bacteria or bacterial groups enumerated with the phylogenetic FISH probes were observed in the cellulose non-prebiotic fermentations. Numbers of total bacteria significantly increased in all other fermentations (P < 0·05). Significant increases after 24 h occurred in the Bifidobacterium genus in the presence of oligofructose (P = 0·02), jumbo porridge oats (P = 0·03), instant porridge (P = 0·03), granola (P = 0·02) and 100 % wholegrain aggregate (P = 0·03). Oligofructose (P = 0·03) fermentation resulted in significant increases in the Coriobacterium–Atopobium cluster enumerated by the Ato291 probe after 5 h. Jumbo porridge oats (P = 0·03) and granola (P < 0·01) resulted in significant increases in the Atopobium cluster after 10 h. Granola (P = 0·01), 100 % wholegrain aggregate (P < 0·01), instant porridge (P ≤ 0·01) and 70 % wholegrain loops (P = 0·01) produced a significant increase in the Bacteroides–Prevotella group after 10 h of fermentation. Granola (P = 0·01) fermentation resulted in a significant increase in the Lactobacillus–Enterococcus group after 24 h, while 100 % wholegrain aggregate (P = 0·04) and 70 % wholegrain loops (P = 0·01) resulted in significant increases after 10 h. Granola (P < 0·01) and instant porridge (P = 0·04) resulted in a significant increase in the Clostridium histolyticum subgroup after 24 h fermentation. Porridge (P < 0·01) and 100 % wholegrain aggregate (P = 0·03) fermentation resulted in a decrease in the Clostridium histolyticum subgroup after 24 h. No significant changes in the Ruminococcus–Eubacterium–Clostridium cluster were observed for any of the fermentations.
Table 2 Bacterial populations (log10 cells/ml batch culture fluid) in pH-controlled and stirred batch cultures at 0, 5, 10 and 24 h using oligofructose as a positive control to compare the microbiota-modulating abilities of different processed breakfast cereals (Mean values with their standard errors)

JPO, jumbo porridge oats, IP, instant porridge, 100 %WGA, 100 % wholegrain aggregate, 70 % WGL, 70 % wholegrain loops.
Mean value was significantly different from that at 0 h: * P < 0·05, ** P < 0·001.
Comparison of treatment effects on bacterial group populations over 24 h fermentation
No significant differences in the changes in the Bacteroides–Prevotella or Lactobacillus–Enterococcus groups were observed among treatments. Changes in Bifidobacterium, Atopobium and Clostridium histolyticum populations compared with cellulose were significantly higher for all test substrates (P < 0·01) at 24 h. The increase seen in Clostridium histolyticum population after instant porridge fermentation was significantly higher compared with the decrease in this population observed after jumbo porridge oats (P = 0·04) and 100 % wholegrain aggregate (P = 0·04) fermentation. Jumbo porridge oats (P < 0·01) and 100 % wholegrain aggregate (P < 0·01) fermentations were again significantly lower for the Clostridium histolyticum profile compared with 70 % wholegrain loops fermentation. 100 % Wholegrain aggregate fermentation resulted in a quicker increase in Bifidobacterium compared with granola (P < 0·01). However, granola (P < 0·01) and oligofructose (P < 0·01) had a higher increase in Bifidobacterium compared with jumbo porridge oats. The changes seen in Atopobium populations after oligofructose fermentation were significantly higher than the changes in populations observed after 70 % wholegrain loops (P = 0·01) and 100 % wholegrain aggregate (P < 0·01) fermentation. The increase seen after jumbo porridge oat fermentation was significantly higher compared with 100 % wholegrain aggregate (P = 0·01) for the Atopobium population. A significant increase among treatments was observed for cellulose compared with granola (P < 0·01) and jumbo porridge oats (P < 0·01) for Ruminococcus–Eubacterium–Clostridium cluster changes. The total bacteria population was significantly higher when oligofructose was compared with 100 % wholegrain aggregate (P < 0·01) and granola (P = 0·01).
SCFA production
Changes in SCFA concentrations after 0, 5, 10 and 24 h fermentation with the different test substrates are shown in Table 3. A significant increase in total (acetic, propionic and butyric acids combined) SCFA concentration after 10 h fermentation by the faecal microbiota (P < 0·05) was observed with all substrates tested. Acetic acid was the dominant SCFA produced in all fermentations and a significant increase in production of this SCFA was observed for cellulose (P = 0·02) and 100 % wholegrain aggregate (P < 0·01) after 10 h incubation, while for 70 % wholegrain loops (P < 0·01) and refined flakes instant porridge (P = 0·03) the concentration of acetate increased significantly after 5 h. However, a significant increase in acetate was observed after 24 h for oligofructose (P < 0·01), jumbo porridge oats (P < 0·01) and granola (P < 0·01). Propionate increased significantly after 10 h fermentation for both 70 % wholegrain loops (P < 0·01) and 100 % wholegrain aggregate (P < 0·01), while oligofructose (P < 0·01), cellulose (P = 0·02), jumbo porridge oats (P = 0·01) and granola (P < 0·01) resulted in significant propionate production only after 24 h. Instant porridge fermentation resulted in a significant increase after 5 h (P = 0·03). A significant increase in butyrate was recorded after 10 h fermentation for 70 % wholegrain loops (P = 0·03) and significant (P < 0·01) production was detected after 24 h for all other substrates except cellulose.
Table 3 SCFA concentrations (mmol/l) in batch cultures at 0 (inoculum), 5, 10 and 24 h using different processed breakfast cereals, as compared with oligofructose as a positive control (Mean values with their standard errors)

JPO, jumbo porridge oats, IP, instant porridge, 100 %WGA, 100 % wholegrain aggregate, 70 % WGL, 70 % wholegrain loops.
* Mean value was significantly different from that at 0 h (P < 0·05).
† Mean value was significantly different from that at 5 h (P < 0·05).
‡ Mean value was significantly different from that at 10 h (P < 0·05).
Glycaemic index of cereal products
All participants completed the study. Their glycaemic responses to all cereals were in the ‘normal’ range for non-diabetic subjects. The mean glycaemic response curves of the test cereals and the two glucose references are shown in Fig. 1. The GI values were: 100 % wholegrain aggregate, 40 (sem 22·6)%; granola, 44 (sem 12·9)%; jumbo porridge oats, 50 (sem 11·9)%; 70 % wholegrain loops, 74 (sem 13·8)%; instant porridge, 88 (sem 13·9)%. The GI values for granola (P < 0·01) and 100 % wholegrain aggregate (P = 0·03) were significantly lower than that for 70 % wholegrain loops. No significant difference was observed between the 25 g glucose reference and instant porridge, jumbo porridge oats or among refined flakes instant porridge and jumbo porridge oats or granola.

Fig. 1 Fasting and postprandial capillary blood glucose responses to the test cereals over 120 min. The values at different time points were based on twenty-four blood samplings for each glucose reference and on twelve blood samplings for test foods. Data from twelve volunteers were averaged. Values are means, with standard errors represented by vertical bars. (–◇–), 50 g Glucose; (–□–), 25 g glucose; (–Δ–), jumbo porridge oats; (–X-), instant porridge; (–*–), 100 % wholegrain aggregate; (–○–), granola; (–+–), 70 % wholegrain loops.
Discussion
Wholegrain cereal consumption has been linked to reduction in a number of chronic diseases of the Western population including diabetes, obesity and CVD( Reference Steffen, Jacobs and Stevens 28 – Reference Leeds 30 ). The aim of the present study was to investigate the impact of five wholegrain breakfast cereals containing different grains, and processed to different extents, on fermentation profiles within the human gut microbiota, compared with the confirmed prebiotic, oligofructose, and to determine the cereal's GI values to allow investigation of combined wholegrain potential mechanisms of protection.
The data from the present study further demonstrated that extensively processed wholegrain cereals resulted in higher GI responses compared with minimally processed wholegrain cereals (i.e. jumbo porridge oats, granola and 100 % wholegrain aggregate compared with 70 % wholegrain loops and instant porridge). In addition, processing of the wholegrain cereals affected the microbiota-modulating activity of the non-digestible components of the breakfast cereals. This has important implications in relation to dietary therapy and cardiometabolic risk reduction and for the definition of a wholegrain product.
In the present study, fermentation of the non-digestible components resulting from in vitro digestion of five commonly consumed breakfast cereals was studied. A large effect on the fermentation profile in relation to changes of the dominant members of the gut microbiota and metabolites produced was reported. Significant increases in the growth of the Bifidobacterium genus and the Lactobacillus–Enterococcus group were observed in the case of granola, 100 % wholegrain aggregate and 70 % wholegrain loops, which supports previous in vivo and in vitro studies using wheat, maize and oat grain breakfast cereals( Reference Costabile, Klinder and Fava 13 , Reference Carvalho-Wells, Helmolz and Nodet 14 , Reference Bornet, Brouns and Tashiro 18 ). Although the non-digestible components of the cereals involved in the observed microbiota changes have not been identified, we postulate that RS is probably a key constituent. Previously it has been documented by Livesey et al. that the physical form of barley grain influences the digestion of its starch content( Reference Livesey, Wilkinson and Roe 31 ). This study observed that only 2 % of starch remained undigested after finely milled barley was consumed compared with 17 % after flaked barley was eaten. After flaked barley digestion it was observed that some oligosaccharides persisted into the colon, but largely the undigested carbohydrate was intact unpitted starch granules bound by intact cell walls. It was concluded that possibly the botanical source of cereals and processing, other than retrogradation of starch, are important determinants of starch digestibility and energy value. In two previous studies we documented that the oat flake size and toasting of wheat flakes significantly affected gut microbiota modulation and activity( Reference Connolly, Lovegrove and Tuohy 19 , Reference Connolly, Lovegrove and Tuohy 32 ). Altering the oat flake size had a negative impact on gut microbiota modulation, with the smaller flakes resulting in less prebiotic ability than the larger flakes( Reference Bornet, Brouns and Tashiro 18 ). Scanning electron microscopy was used to examine the physical structure of wheat flakes before, during and after the toasting process. It was observed that the number of intact starch granules decreased and that the structure of the grain was significantly disrupted by this common process( Reference Livesey, Wilkinson and Roe 31 ). The results from our previous investigations along with the present study correspond with the conclusion made by Livesey et al. in that botanical structure and the effect processing has on structure will make an impact on digestibility of carbohydrates and in turn the potential to alter gut microbiota populations and activity.
Acetate is the most abundant SCFA produced in the colon while propionate and butyrate are usually produced in roughly equal proportions( Reference Roediger 16 ). In the present study all cereal fermentation resulted in significant increases in acetate and propionate concentrations. Previous studies have reported different SCFA profiles after fermentation of non-digestible components of wholegrains (for example, β-glucan and arabinoxylan)( Reference Hughes, Shewry and Gibson 33 , Reference Hughes, Shewry and Li 34 ). To date only two studies have investigated the impact of feeding wholegrain breakfast cereals (wheat and maize) on microbiota( Reference Costabile, Klinder and Fava 13 , Reference Carvalho-Wells, Helmolz and Nodet 14 ). These studies did not detect any changes in faecal SCFA concentrations, which is not wholly surprising, as SCFA are readily absorbed in the colon. Butyrate plays a major role in colonocyte proliferation and differentiation( Reference Chai, Evdokiou and Young 35 ), with 70–90 % metabolised by the gut wall cells. Its production has been linked to inhibiting cancer development and has been shown to inhibit cell proliferation( Reference Scheppach and Weiler 36 ). Another potential mechanism of action is that the drop in pH as a result of increased total SCFA production decreases the solubility of free bile acids which, in turn, decreases secondary bile acids that have potential tumour-promoter activity( Reference Cheah and Bernstein 37 ).
RS is a dietary fibre component of wholegrains and its fermentation commonly results in butyrate production. Dietary RS intakes and faecal butyrate levels are seen to be elevated in populations at low risk of diet-related large-bowel diseases( Reference Brouns, Kettlitz and Arrigoni 38 ). This raises the possibility that greater RS consumption could be of health benefit. RS has most recently been suggested as a prebiotic by Bird et al., as most forms stimulate specific beneficial bacteria, resulting in elevated total SCFA and butyrate concentrations and a consequent benefit to the host( Reference Bird, Conlon and Christophersen 39 ). Furthermore, cross-feeding may also occur between bifidobacteria and butyrate-producing colonic bacteria, which may explain the significant increase in acetate (from bifidobacteria) and butyrate production seen in the present study( Reference De Vuyst and Leroy 40 ).
In conclusion, the present study has shown that consumption of wholegrain breakfast cereals has the potential to beneficially modulate the gut microbiota. Significant growth of the Bifidobacterium genus and, in the case of 100 % wholegrain aggregate, granola and 70 % wholegrain loops, Lactobacillus–Enterococcus group was observed. In addition, the GI of the highly processed cereals was greater than the minimally processed cereals. The 100 % wholegrain oat aggregate cereal had the greatest prebiotic potential and lowest GI which, if consumed, could result in cardiometabolic benefit. However, to determine the impact of breakfast cereals with low GI and prebiotic traits on cardiometabolic risk, appropriately powered, randomly controlled human dietary intervention studies are required.
Acknowledgements
The present study was supported by the Reading Endowment Trust Fund (RETF) and The Jordans & Ryvita Company Limited (Holme Mills, Biggleswade, SG18 9JY, UK). M. L. C., J. A. L. and K. M. T. designed the study; M. L. C. drafted the manuscript and all authors read, commented on and approved the submitted version. J. A. L. sits on a number of British Government advisory committees including the Scientific Advisory Committee for Nutrition (SACN). Funds for research studies and studentships have been supplied by Unilever and Sugar Nutrition UK. Between October 1999 and May 2010, K. M. T. worked in the Department of Food and Nutritional Sciences, University of Reading. Funds for research studies and studentships have been supplied by Cereal Partner's UK, Nestlé, Beneo-Orafti, Müller Dairies, Bayer, Unilever, Danisco, Yakult, GSK and Novartis. K. M. T. now works at Fondazione Edmund Mach, Italy, a publicly funded research institute and receives funding from local government and the Marie-Curie Foundation. M. L. C. has no conflicts of interest.








