The importance of dietary Mn for poultry has been well established( Reference Wilgus, Norris and Heuser 1 , Reference Wang, Wang and Wang 2 ). The dietary Mn requirements are higher for broilers than for mammals, and Mn concentrations in common feeds are low( Reference Attia, Qota and Bovera 3 ). Therefore, Mn additives are usually used in broiler diets for optimal growth( Reference Attia, Qota and Bovera 3 ). The parameters selected to estimate nutrient requirements are important, as the maximum responses for various criteria are different( Reference Huang, Lu and Luo 4 ). The National Research Council( 5 ) has recommended the Mn requirement for broilers from 22 to 42 d of age to be 60 mg/kg diet, which was mainly based on studies carried out more than 70 years ago. In addition, in most of these studies, semi-purified diets were used( Reference Wilgus, Norris and Heuser 1 , Reference Insko, Lyons and Martin 6 , Reference Gallup and Norris 7 ), and the parameters selected were growth performance and incidence of leg disease( Reference Gallup and Norris 7 , Reference Southern and Baker 8 ). Using semi-purified diets could remove confounding effects of natural and variable Mn in practical feedstuffs; however, the requirements determined under semi-purified diets might not be applicable to conventional diets because of the absence of phytate and fibre( Reference Halpin and Baker 9 – Reference Baker and Halpin 12 ). Phytate and fibre could inhibit Mn absorption by binding Mn in the intestinal tract, and thus decreasing Mn bioavailability. In fact, Mn is an essential component of Mn-containing superoxide dismutase (MnSOD), which plays an important role in the detoxification of superoxide free radicals. In our laboratory, Luo et al.( Reference Luo, Su and Huang 13 ) reported that MnSOD activity in the heart and Mn concentrations in tissues (heart, liver, kidney and pancreas), especially the former, were sensitive criteria to estimate the Mn requirements of broilers. On the basis of these parameters, the dietary Mn requirements for broilers from 1 to 28 d of age should be 120 mg/kg( Reference Luo, Su and Huang 13 ). Recently, in our laboratory, heart MnSOD mRNA level has been used as a quicker and more consistent biomarker than the above-mentioned parameters to estimate dietary Mn requirement in broilers from 1 to 21 d of age( Reference Li, Lin and Lu 14 ), and 130 mg Mn/kg of diet was required for the maximal expression of this biochemical marker( Reference Li, Lin and Lu 14 ). Later, Li et al.( Reference Li, Lu and Hao 15 ) reported that dietary Mn could modulate the expression of the MnSOD gene in the heart of broilers by altering DNA-binding activities of specificity protein 1 (Sp1) and activating protein-2 (AP-2) at the transcriptional level. However, dietary Mn requirements for expressions of MnSOD mRNA or protein and the DNA-binding activities of the two transcription factors (Sp1 and AP-2) in the heart of broilers from 22 to 42 d of age have not been investigated.
Therefore, the objective of this study was to estimate dietary Mn requirements of broiler chickens fed a conventional maize–soyabean meal diet from 22 to 42 d of age by determining MnSOD activity and its mRNA and protein expression levels, as well as the DNA-binding activities of two transcription factors (Sp1 and AP-2) in the heart of broilers at 42 d of age.
Methods
Animals, diets and experimental design
All experimental procedures were approved by the Office of the Beijing Veterinarians. The management of the Arbor Acres male broiler chickens was in agreement with the guidelines approved by the Arbor Acres Breeding Company in Beijing. The broilers were housed in electrically heated, thermostatically controlled, stainless steel cages (length 100 cm×width 50 cm×height 45 cm) equipped with fibreglass feeders and waterers. They were maintained on a 24-h constant light schedule, and allowed ad libitum access to experimental diets and tap water containing no detectable Mn. Broilers were vaccinated with Newcastle disease vaccine at 7 and 24 d of age, and with infectious bursal disease virus vaccine and bronchitis virus vaccine at 14 and 21 d of age, respectively.
During 1–21 d, the chickens were fed a maize–soyabean meal-based mash diet (Table 1), containing 127 mg Mn/kg of diet by analysis, which was formulated to meet or exceed the requirements of the broilers for all nutrients. About 130 mg Mn/kg of diet was decided on the basis of the result of Li et al.( Reference Li, Lin and Lu 14 ) in our laboratory.
Table 1 Ingredients and chemical composition of the basal diet for broilers, as-fed basis
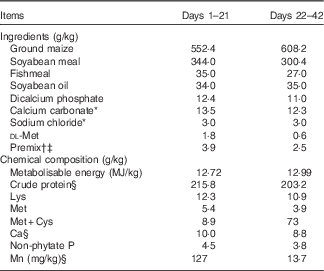
* Feed grade before day 21 of age and reagent grade after day 21 of age.
† Provided per kilogram of diet during 1–21 d (mg/kg): retinyl acetate, 4·12; cholecalciferol, 0·11; dl-α-tocopheryl acetate, 16; menadione, 2; thiamin, 1; riboflavin, 8·5; pyridoxine, 2; vitamin B12, 0·01; pantothenic acid, 10; niacin, 32·5; folic acid, 1; biotin, 0·16; choline, 700; Cu, 8; Zn, 60; Fe, 80; I, 0·35; Se, 0·15.
‡ Provided per kg of diet during 22–42 d (mg/kg): retinyl acetate, 2·48; cholecalciferol, 0·06; dl-α-tocopheryl acetate, 9·6; menadione, 1·2; thiamin, 0·6; riboflavin, 5·1; pyridoxine,1·2; vitamin B12, 0·006; pantothenic acid, 6; niacin,19·5; folic acid, 0·6; biotin, 0·09; choline, 500; Cu, 8; Zn, 60; Fe, 80; I, 0·35; Se, 0·15.
§ Values determined by analysis. Each value is based on triplicate determinations.
At 22 d of age, a total of 384 chickens were randomly divided into one of eight treatment groups with eight replicate cages (6 chickens/cage) for each treatment in a completely randomised design.
Broilers were fed a maize–soyabean meal-based mash diet supplemented with 0, 20, 40, 60, 80, 100, 120 or 140 mg Mn/kg as Mn sulphate (reagent grade MnSO4.H2O) for 21 d. The basal diet met the requirements for all nutrients( 5 ) except for Mn (Table 1). The dietary Mn contents for the eight treatments were 13·7, 32·0, 49·8, 71·0, 90·3, 111, 128 and 147 mg/kg by analysis on an as-fed basis, respectively.
At 28, 35 and 42 d of age, chicken weight and feed intake (FI) were recorded to calculate daily weight gain (G), FI and gain:feed ratio (G:F) during the 21-d study period. Incidence of leg abnormality was calculated as a percentage of chickens with visual swelling at the tibiotarsus joint within each cage weekly( Reference Li, Lin and Lu 14 ).
Sample collections
At 42 d of age, sixteen chickens (two chickens from each replicate cage) from each treatment group were killed by cervical dislocation. The heart was excised immediately. A set of heart sub-samples was snap-frozen in liquid N2 and then stored at −80°C for analyses of MnSOD mRNA and protein expression levels and Sp1 and AP-2 DNA-binding activities, whereas another set of sub-samples was kept on ice and stored at −20°C for subsequent determination of Mn contents and MnSOD activities. To avoid regional variation within the heart tissue, the same region of the heart was collected for the analysis of each parameter. To reduce individual biological variation, samples from two chickens in each replicate cage were pooled into one sample of equal weight before analyses, and thus there were a total of eight replicate samples for each treatment.
Analyses of manganese contents
Contents of Mn in tap water, diets and heart tissue were determined by inductively coupled plasma emission spectroscopy (Thermo Fisher Scientific) as described by Li et al.( Reference Li, Luo and Liu 16 ). Approximately 2 ml of tap water, or 0·5 g of feed or 0·6 g of tissue was weighed in triplicate and digested with 8 ml (tap water) or 10 ml (feed and tissue) of HNO3 and 0·4 ml of HClO4 at 200°C in a 50-ml calibrated flask until the solution cleared and evaporated to almost dryness; the solutions were diluted 20-fold for feed samples and 25-fold for tissue samples with 2 % HNO3 before analysis. Validation of the mineral analysis was conducted using bovine liver powder (GBW (E) 080193; National Institute of Standards and Technology) as a standard reference material (SRM). Approximately 0·2 g of bovine liver powder was weighed and digested as described above, and was diluted 50-fold with 2 % HNO3 before analysis. The actual Mn recovery rate for the bovine liver SRM was determined to be about 99 % in the present study.
Heart manganese-containing superoxide dismutase activity assay
Heart MnSOD activity was measured by the nitrite method as previously described( Reference Li, Luo and Liu 16 ). Duplicate samples (approximately 0·6 g) of heart tissue were homogenised in 6 ml of 0·9 % cold saline, and then sonicated at 4°C for 1 min (1 s with 2-s interval; JY92-11; Ningbo Xinzhi Bio-technology Co., Ltd). The heart homogenate was centrifuged at 1500× g for 15 min, and the supernatant was diluted 50-fold with deionised H2O for MnSOD activity analysis. The MnSOD activity in the heart was expressed as nitrite units (NU) per gram of fresh weight, and 1 NU was defined as the amount of enzyme needed to obtain 50 % inhibition of nitrite formation.
RNA extraction and quantitative RT-PCR
Total RNA in heart tissue was isolated using Trizol reagent (Invitrogen Life Technologies) according to the manufacturer’s protocol. Concentrations of total RNA were analysed by measuring UV light absorbance at 260 nm with a spectrophotometer (ND-100; NanoDrop Technologies). Complementary DNA was synthesised using the SuperScript_III First-Strand Synthesis for RT-PCR kit (cat no. 18080-051; Invitrogen) with DNA Engine PCR instrument (Bio-RAD). Quantitative PCR was performed in triplicate on an Abi Prism 7500 apparatus (Applied Biosystems) according to optimised PCR protocols( Reference Li, Lu and Hao 15 ). The primers for MnSOD (forward, 5′-CACTCTTCCTGACCTGCCTTAC-3′; reverse, 5′-TAGACGTCCCTGCTCCTTATTA-3′) and reference gene β-actin (forward, 5′-CAGCTACGTTGGTGATGAAGCC-3′; reverse, 5′-CAAGAAAGATGGCTGGAAGAGG-3′) were used for the amplification reactions, respectively. We used the relative standard curve method to quantify gene expression, as previously described( Reference Li, Lu and Hao 15 ). The results are expressed as the ratio of MnSOD mRNA abundance:β-actin mRNA abundance.
Measurements of specificity protein 1 and activating protein-2 DNA-binding activities
The DNA-binding activities of transcriptional factors Sp1 and AP-2 were analysed using the electrophoretic mobility shift assay (EMSA)( Reference Li, Lu and Hao 15 ). Minced tissue samples were homogenised in HEPES buffer and incubated on ice for 30 min. After the addition of Nonidet P-40 to a final concentration of 5 g/l, the homogenate was vortexed vigorously for 15 s and centrifuged at 14 000× g for 1 min at 4°C. The pellet was incubated with HEPES buffer containing 420 mmol/l NaCl for 30 min on ice. The nuclear extract supernatant was obtained by centrifugation at 12 000× g for 5 min at 4°C. EMSA was performed using consensus sequences for Sp1 (59-CGAGGAGGGGCGGGCGTGTGAG-39) and AP-2 (59-GCAGTGCGGGCGCAGGCGCGG-39). The complementary single-stranded oligonucleotide was end-labelled separately using biotin-11-dUTP (deoxyuridine triphosphate) and terminal deoxynucleotidyl transferase according to instructions of the biotin 3′ end DNA labelling kit (Pierce) and then annealed before being used in the binding reactions. The binding specificity between Sp1 or AP-2 protein and its respective consensus sequences were verified by competition and supershift assay. Samples were then subjected to a 6 % non-denaturing PAGE in Tris borate glycine buffer and transferred to a nitrocellulose membrane. The biotin-labelled DNA–protein complex was detected using streptavidin–horseradish peroxidase conjugate and chemiluminescent substrate according to instructions of the Lightshift Chemiluminescent EMSA kit (Pierce). The data are expressed as densitometry units of the DNA–protein complex in each experimental group.
Western blotting assay
The heart samples were homogenised and cleaved using radio immunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology), and then sonicated at 4°C for 2 min (2 s with 10-s intervals). After centrifugation at 12 000× g for 5 min at 4°C, the supernatants were collected for SDS-PAGE and Western blot analysis. Total protein contents in the supernatants were determined by Bradford Protein Assay Kit (Beyotime Institute of Biotechnology). Other procedures for Western blot analysis were performed as described by Li et al.( Reference Li, Lu and Hao 15 ).
Statistical analyses
The effect of dietary Mn treatment was analysed by one-way ANOVA using the general liner model procedure of SAS (version 8.0; SAS Institute Inc.). Differences between means were assessed by Duncan’s multiple tests. Cage was the experimental unit. Percentage data for incidence of leg abnormality were transformed to arcsine for analysis. Orthogonal comparisons were applied for linear and quadratic responses of dependent variables to independent variables. Regression analyses were used to estimate Mn requirements (the inflection point from a broken-line model, or the maximum response from a quadratic model) whenever the response was significant (P<0·05)( Reference Li, Lin and Lu 14 , Reference Jiang, Lu and Li 17 ).
Results
Growth performance and incidence of leg abnormality
The G, FI and G:F were not affected (P>0·05) by dietary Mn concentration (data not shown). Incidence of leg abnormality tended to decrease with increasing dietary Mn concentrations, although treatment differences were not significant (P>0·05) (data not shown).
Heart manganese content
Dietary Mn had an effect (P<0·01) on Mn content in the heart (Table 2). Heart Mn content increased linearly (P<0·001) with increasing dietary Mn concentrations.
Table 2 Effect of dietary manganese concentration on manganese content, manganese-containing superoxide dismutase (MnSOD) mRNA and protein expression levels and MnSOD activity in the heart of broilers at 42 d of age (Mean values with their pooled standard errors, n 8)
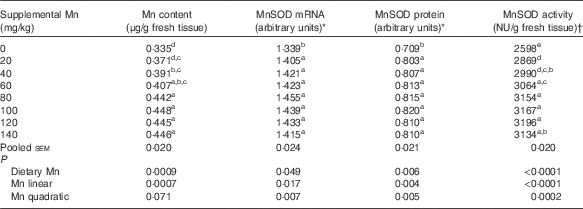
a,b,c,d,e Mean values within a column with unlike superscript letters were significantly different (P<0·05).
* Expressed as the ratio of MnSOD mRNA abundance or MnSOD protein band intensity:β-actin mRNA or β-actin protein.
† One nitrite unit (NU) was defined as the amount of enzyme needed to obtain 50 % inhibition of nitrite formation.
Heart manganese-containing superoxide dismutase activity
Dietary Mn concentration significantly affected (P<0·0001) heart MnSOD activity (Table 2). Heart MnSOD activity increased linearly (P<0·0001) and quadratically (P<0·001) as dietary Mn concentrations increased, and reached a plateau visually at about a supplemental Mn concentration of 80 mg/kg.
Heart manganese-containing superoxide dismutase mRNA
Heart MnSOD mRNA was significantly affected (P<0·05) by dietary Mn treatment, and increased linearly (P<0·05) and quadratically (P<0·01) with increasing dietary Mn concentrations (Table 2). The highest MnSOD mRNA expression level was observed visually at about a supplemental Mn concentration of 80 mg/kg.
Heart manganese-containing superoxide dismutase protein expression level
Dietary Mn had a significant effect (P<0·01) on heart MnSOD protein expression levels (Table 2). As dietary Mn concentrations increased, linear and quadratic (P<0·01) responses were observed in MnSOD protein expression levels with the highest MnSOD protein expression level visually at about a supplemental Mn concentration of 100 mg/kg.
DNA-binding activity of specificity protein 1
Dietary Mn significantly affected (P<0·01) the DNA-binding activity of Sp1 in the heart (Table 3). With increasing dietary Mn concentrations, DNA-binding activities of Sp1 in the heart showed a linear response (P<0·0001) to dietary Mn concentrations.
Table 3 Effect of dietary manganese concentration on the DNA-binding activities of specificity protein 1 (Sp1) and activating protein-2 (AP-2) in the heart of broilers at 42 d of age (Mean values with their pooled standard errors, n 8)
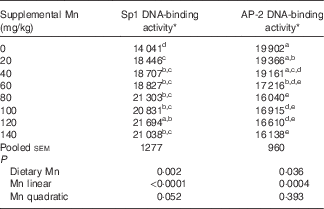
a,b,c,d,e Mean values within a column with unlike superscript letters were significantly different (P<0·05).
* Data are expressed as densitometry units of the RNA–protein complex in each experimental treatment.
DNA-binding activity of activating protein-2
Dietary Mn significantly affected (P<0·05) the DNA-binding activity of AP-2 in the heart (Table 3). As dietary Mn concentrations increased, the DNA-binding activities of AP-2 in the heart decreased linearly (P<0·001).
Estimations of the dietary manganese requirements of broilers
Results of dietary Mn requirements of broilers as estimated by the non-linear regression analyses are shown in Table 4. On the basis of a broken-line model (P<0·01) of heart MnSOD activity and quadratic models of heart MnSOD mRNA (P<0·001) and protein (P<0·05) expression levels with dietary Mn concentrations, dietary Mn requirements for the full expression of heart MnSOD activity and mRNA and protein expression levels were 94, 101 and 104 mg/kg, respectively for broiler chickens fed a conventional maize–soyabean meal diet from 22 to 42 d of age. These requirement values are higher than those (80, 80 and 100 mg/kg) derived by visual approximation from the tabulated data, but more accurate and valid than the latter as they were derived by statistical evaluation of the regression models.
Table 4 Estimations of dietary manganese requirements based on non-linear regressions of heart manganese-containing superoxide dismutase (MnSOD) mRNA and protein expression levels and MnSOD activity on dietary supplemental manganese concentrations
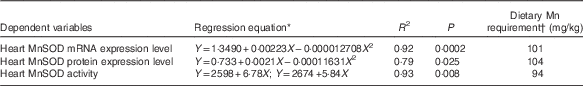
* Y is the dependent variable and X the supplemental Mn concentration (mg/kg).
† Dietary Mn requirement=the optimal supplemental Mn concentration+the analysed Mn in the basal diet (13·7 mg/kg).
Discussion
In earlier studies, growth performance and incidence of leg abnormality were often used to assess Mn requirements in chickens fed semi-purified diets( Reference Gallup and Norris 7 , Reference Southern and Baker 8 ). However, growth performance was usually not sensitive when chickens were fed a conventional maize–soyabean meal diet( Reference Attia, Qota and Bovera 3 , Reference Luo, Su and Huang 13 , Reference Li, Lu and Hao 15 ). Incidence of leg weakness could not be completely prevented by Mn supplementation( Reference Lu, Ji and Luo 18 ), and was also not a sensitive criterion for estimation of Mn requirement( Reference Li, Lin and Lu 14 ). The results from the current study are consistent with those from previous studies, illustrating that growth performance and incidence of leg abnormality were not suitable parameters to estimate Mn requirements of broilers when a maize–soyabean meal diet is used.
Numerous studies have indicated that heart Mn concentration is a sensitive indicator of Mn deficiency in animals( Reference Luo, Su and Huang 13 , Reference Black, Ammerman and Henry 19 , Reference Pallauf, Kauer and Most 20 ). A study with lambs demonstrated that heart Mn could reflect the bioavailability of Mn sources because it showed a good linear response to dietary supplemental Mn (500–4000 mg/kg)( Reference Black, Ammerman and Henry 19 ). Similar results in broilers were obtained by Li et al.( Reference Li, Luo and Liu 16 , Reference Li, Luo and Lu 21 ) in our laboratory. The linear response of heart Mn to dietary Mn in the present study indicates that heart Mn is not a useful marker for the assessment of Mn requirement of broilers.
As a Mn-dependent enzyme, MnSOD activity is typically reduced by Mn deficiency in animals( Reference Li, Luo and Liu 16 , Reference Pallauf, Kauer and Most 20 , Reference Zhu, Lu and Li 22 ). Luo et al.( Reference Luo, Su and Huang 13 ) found that heart MnSOD activity was a sensitive criterion for Mn requirement estimation of broilers from 1 to 28 d. Li et al.( Reference Li, Lin and Lu 14 ) observed a quadratic curve for heart MnSOD activity relative to dietary Mn concentration, and that the full activity expression of MnSOD in the heart of broilers from 1 to 21 d occurred at 135–156 mg Mn/kg of diet. Our results showed that dietary Mn had a broken-line effect on heart MnSOD activity, and 94 mg Mn/kg was required by broilers from 22 to 42 d of age based on this parameter. Therefore, dietary Mn needs for the full expressions of heart MnSOD activity in broilers showed a substantial decrease with increasing age of chickens.
The MnSOD gene in the heart of broilers is regulated transcriptionally in a Mn-dependent manner( Reference Li, Lu and Hao 15 , Reference Li, Luo and Lu 21 , Reference Li, Luo and Lu 23 , Reference Luo, Li and Lu 24 ). Mn deficiency in mice resulted in low Mn concentration, low MnSOD activity and low MnSOD mRNA in the liver( Reference Borrello, De Leo and Galeotti 25 ). Li et al.( Reference Li, Lu and Hao 15 ) also reported that dietary Mn could modulate MnSOD gene expression in heart tissue of broilers at the transcriptional level. In addition, they found that the heart MnSOD mRNA level could be used for a quicker and consistent evaluation of dietary Mn requirement of broilers from 1 to 21 d of age( Reference Li, Lin and Lu 14 ). The results from the current study indicated that MnSOD mRNA levels in the heart increased quadratically as dietary Mn concentrations increased, and heart MnSOD mRNA level was a sensitive marker to assess Mn status and Mn requirements for broilers from 22 to 42 d of age. Therefore, our findings are in line with the observations of previous studies. On the basis of the heart MnSOD mRNA level, dietary Mn requirement for broilers from 22 to 42 d of age was 101 mg Mn/kg of diet, which was lower than the Mn requirement value (130 mg Mn/kg of diet) for broilers from 1 to 21 d of age in the study of Li et al.( Reference Li, Lin and Lu 14 ).
Early researchers demonstrated that the promoter sequences of the chicken MnSOD gene contained multiple Sp1 and AP-2 binding sites, and the transcription of the MnSOD gene might be controlled by these regulatory elements( Reference DiSilvestre, Kleeberger and Johns 26 , Reference Xu, Porntadavity and St Clair 27 ). The results from the present study indicated that Sp1 DNA-binding activities increased and AP-2 DNA-binding activities decreased linearly as dietary Mn concentrations increased. The enhanced Sp1 DNA-binding activities and decreased AP-2 DNA-binding activities, as well as increased MnSOD mRNA levels, due to dietary Mn additions revealed that Sp1 up-regulated and AP-2 down-regulated MnSOD gene expression in broilers, respectively, as previously reported by Li et al.( Reference Li, Lu and Hao 15 ). The above results also suggest that the DNA-binding activities of Sp1 and AP-2 might be sensitive indices to assess Mn status and bioavailability, but may not be suitable indices to access Mn requirements for broilers.
Clerch et al.( Reference Clerch and Massaro 28 ) demonstrated that during exposure to hyperoxia (>95 % O2), the lungs of rats were substantially damaged, and lung MnSOD activity dropped by approximately 50 %. They also found that the fall in lung MnSOD activity was not due to a failure to increase MnSOD mRNA, but rather due to impaired translational efficiency( Reference Clerch and Massaro 28 , Reference Clerch, Massaro and Berkovich 29 ). These previous studies imply that the synthesis of MnSOD protein is essential for tolerance to hyperoxia in animals. The results from the present study illustrated that the addition of Mn to broiler diets increased the synthesis of MnSOD protein, and thus might enhance the ability of broilers to resist the damage caused by oxygen free radicals and other stress factors. Until now, information is still limited regarding the translational regulations of MnSOD gene expression in chickens by dietary Mn. Li et al.( Reference Li, Lu and Hao 15 ) found that chickens fed Mn-supplemented diets had higher MnSOD protein concentrations in heart than those fed Mn-deficient diets, suggesting that dietary Mn could modulate MnSOD gene expression in heart tissue of broilers at the translational level. Furthermore, they demonstrated that addition of Mn elevated MnSOD protein expression level and its activities in the primary broiler myocardial cells( Reference Li, Lu and Liao 30 ). Our findings highlighted that MnSOD protein concentration was a new and valuable parameter to assess Mn requirement for broilers from 22 to 42 d of age. In the current study, the Mn requirement for the full expression of heart MnSOD protein in broilers from 22 to 42 d of age was 104 mg Mn/kg of diet, which was a little higher than the Mn requirements (101 and 94 mg/kg) for the full expression of heart MnSOD mRNA and activity.
As for the evaluations of nutrient requirements of animals, the indicators used in fitted non-linear regression models with lower P values and higher R 2 values should be more reliable, because lower P values could indicate the significance and validation of fitted non-linear regression models, and higher R 2 values could reflect better correlations between the response criteria and dietary nutrient intakes. The above rationale and approach have been successfully used in previous studies on dietary mineral requirements of broilers( Reference Huang, Lu and Luo 4 , Reference Li, Lin and Lu 14 , Reference Jiang, Lu and Li 17 , Reference Liao, Li and Lu 31 , Reference Liu, Liao and Lu 32 ). In the current study, the fitted non-linear regression model for the heart MnSOD mRNA expression level had the lowest P value (0·0002) and higher R 2 value (0·92); therefore, the heart MnSOD mRNA expression level is a more reliable indicator for the evaluation of Mn requirements of broilers.
In conclusion, the results from the present study indicate that 94 mg Mn/kg is adequate for the full expression of MnSOD activity in the heart of broilers. Full expressions of MnSOD mRNA and protein in the heart of broilers require 101 and 104 mg Mn/kg diet, respectively. In addition, heart MnSOD mRNA expression level is a more suitable and reliable indicator than heart MnSOD activity and MnSOD protein expression level for the assessment of Mn requirement of broilers. It appears that about 100 mg Mn/kg of diet is required for broilers fed the conventional maize–soyabean meal diet from 22 to 42 d of age.
Acknowledgements
The present study was supported by the Key International Cooperation Program of the National Natural Science Foundation of China (grant no. 31110103916; Beijing), the Agricultural Science and Technology Innovation Program (grant no. ASTIP-IAS08; Beijing) and the China Agriculture Research System (grant no. CARS-42; Beijing).
The authors’ contributions are as follows: X. L. and L. L. designed the experiment; L. L. drafted the manuscript; X. L. and R. W. participated in writing and editing of the manuscript; B. C. conducted most of the experiments and analysed the data; L. Z. performed the Mn analysis; X. L. had primary responsibility for the final content. All authors read and approved the final version of the manuscript.
The authors have no personal conflicts of interest to declare.









