Inflammatory bowel disease (IBD), a collective term for the diseases including Crohn's disease and ulcerative colitis (UC), is characterised by the chronic and relapsing inflammation of the gastrointestinal tract( Reference Fiocchi 1 ). UC exclusively affects the colon and rectum at the mucosal level( Reference Head and Jurenka 2 ). It begins in early adulthood and continues throughout life and affects millions of individuals worldwide( Reference Cosnes, Gower-Rousseau and Seksik 3 ). Men seem to be affected slightly more frequently than women( Reference Cosnes, Gower-Rousseau and Seksik 3 ). In recent years, the incidence of UC in traditionally high-incidence areas such as the USA and Europe has become relatively stable. However, this disease has become more prevalent in previously low-incidence areas including Asia( Reference Ng, Tang and Ching 4 ). The crude annual incidence rate in mainland China in 2011–12 was 0·22–2·27/100 000 persons( Reference Ng, Tang and Ching 4 ). Although the exact aetiology of UC remains undetermined, the current leading hypothesis emphasises genetic predisposition to the dysregulation of the gastrointestinal immune system( Reference Loftus 5 ). Although conventional treatments (with aminosalicylates, corticosteroids, antibiotics and immunomodulators) can be effective at maintaining remission and decreasing the length of active disease periods, these are not without side effects( Reference Head and Jurenka 2 ). Consequently, potential adverse events, if not more so, and costs have led investigators to search for novel therapeutic approaches.
There is rapidly growing evidence supporting the protective effects of dietary fibres against the pathogenic process of intestinal inflammation in clinical and experimental colitis( Reference Head and Jurenka 2 ). Dietary fibre is a collective term for a variety of plant substances that are resistant to enzymatic digestion in the upper gastrointestinal tract( Reference Eastwood and Passmore 6 ). The dietary fibre from Plantago ovate has been proven to increase butyrate production, lower TNF-α concentrations and maintain remission in UC( Reference Fernandez-Banares, Hinojosa and Sanchez-Lombrana 7 , Reference Rodríguez-Cabezas, Galvez and Lorente 8 ). Kanauchi et al. ( Reference Kanauchi, Suga and Tochihara 9 , Reference Kanauchi, Iwanaga and Mitsuyama 10 ) reported that germinated barley foodstuff, a protein-rich fibre made from brewer's spent grain, enhances butyrate production, inhibits TNF-α production, prevents mucosal damage and reduces the clinical activity of UC. In recent years, β-glucans, one of the most abundant forms of polysaccharides present in the plant cell walls, have been considered as health-promoting dietary fibres. Limited information exists about the prevention and/or treatment of UC with β-glucans, even though these are common in the food of humans. Current data suggest that β-glucans have the ability to modulate immune response by triggering immune cells through several immune receptors including Dectin-1and complement receptor 3( Reference Chan, Chan and Sze 11 ).
Salecan is a type of β-glucan derived from Agrobacterium sp. ZX09 consisting of the repeating unit → 3)-β-d-Glcp-(1 → 3)-(β-d-Glcp-(1 → 3)-β-d-Glcp-(1 → 3))3-α-d-Glcp-(1 → 3)-α-d-Glcp-(1 → ( Reference Xiu, Kong and Zhou 12 ), and has potential applications in the food industry due to its excellent toxicological profile and rheological properties( Reference Xiu, Zhou and Zhu 13 , Reference Xiu, Zhan and Zhou 14 ). Similar to other β-glucans, Salecan exhibits multiple biological activities in the gastrointestinal tract. Due to its indigestible character and high water-holding capacity, Salecan given intragastrically stimulates small-intestinal transit and improves the output of faeces in experimental constipated mice( Reference Zhou, Jia and Chen 15 ). Dietary Salecan intake has been demonstrated to be effective at decreasing fat absorption and improving glucose tolerance in high-fat diet-fed mice( Reference Zhang, Xia and Pang 16 ). Moreover, results from a recent study suggest that Salecan is preferentially fermented by lactobacilli and bifidobacteria and affects gut microbiota balance to positively direct metabolic activity towards increased colonic production of butyrate( Reference Zhou, Pu and Xia 17 ). The aim of the present study was to assess the protective effects of Salecan in the dextran sulphate sodium (DSS) model of mouse colitis. Special attention was paid to its effect on the production of TNF-α.
Materials and methods
Salecan
Salecan was extracted from the fermentation broth of Agrobacterium sp. ZX09 using centrifugation and ethanol precipitation according to a previously described method( Reference Xiu, Kong and Zhou 12 , Reference Xiu, Zhou and Zhu 13 ). Commercial Salecan (chemical composition: sugar 77·13 %, protein 6·2 %, moisture 5·2 % and ash 10·28 %; average molecular weight 2 × 106; water soluble) was purchased from Karroten Company.
Animals and experimental design
All animal care and use procedures were approved by and in accordance with the Institutional Animal Care and Use Committee at the Nanjing University of Science and Technology. Male C57BL/6J mice at the age of 7 weeks were housed in a temperature- and humidity-controlled room under a 12 h light–12 h dark cycle; they had free access to tap water and food. The mice were randomly assigned to four groups (n 6) as follows (Fig. 1): control group, which was fed the standard diet for 26 d; DSS group, which was fed the standard diet for 26 d plus 4 % DSS in drinking-water (w/v, prepared daily; average molecular weight 36 000–50 000; MP Biomedicals) during the last 5 d of the experimental period; two Salecan-treated groups (LS+DSS and HS+DSS), which were fed standard chow supplemented with 4 and 8 % Salecan, respectively, for 26 d plus 4 % DSS in drinking-water during the last 5 d of the experimental period. The dose of Salecan was selected based on the results of previous studies( Reference Rodríguez-Cabezas, Galvez and Lorente 8 , Reference Zhou, Pu and Xia 17 , Reference Nosál'ová, Bobek and Cerná 18 ). The intake of both food and water per mouse was measured throughout the duration of the study. After the 5th day of colitis induction, the mice were killed under anaesthesia.
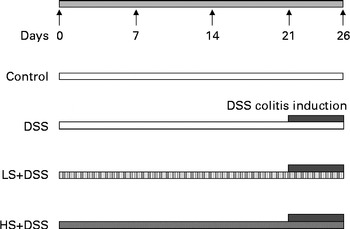
Fig. 1 Patterns for the induction of colitis by dextran sulphate sodium (DSS) in male C57BL/6J mice. The mice were fed a diet containing either 4 % (w/w) or 8 % Salecan (LS or HS, respectively) from 21 d before the start of DSS administration to the day before killing.
Assessment of colonic damage
Colonic damage was monitored daily by an observer who was unaware of the treatment based on a scoring system that evaluates change in body weight, stool consistency, faecal occult blood and overall condition of the mice to provide a disease activity index as described by Murthy et al. ( Reference Murthy, Cooper and Shim 19 ) (Table 1). Faecal occult blood was investigated using Hemoccult II slides (SmithKline Diagnostics Inc.).
Table 1 Criteria for scoring disease activity index*

* Disease activity index = combined score of weight loss, stool consistency and bleeding/3.
† Normal stools, well-formed pellets; loose stools, pasty stools that do not stick to the anus; diarrhoea, liquid stools that stick to the anus.
Sampling procedure
Blood samples were drawn from the mice under anaesthesia and collected in EDTA-treated tubes to determine the haematological profile. Colons were removed, rinsed with ice-cold phosphate buffer solution and blotted dry, and their lengths were measured. Then, the colon of each mouse was divided based on the percentage of total colon length: the proximal 30 % was discarded; the next 30 % was taken for the quantitative determination of mRNA expression; the adjacent 10 % was fixed for histological examination; the remaining 30 % was snap-frozen in liquid N2 for myeloperoxidase (MPO) activity assay( Reference Reardon, Sanchez and Hogaboam 20 ). The weights of the spleens were also recorded.
Haematological analysis
Haematological parameters were determined using an automated haematology analyser. The parameters analysed were erythrocytes, Hb, haematocrit, mean corpuscular volume, mean corpuscular Hb, mean corpuscular Hb concentration, leucocyte count, lymphocyte count and neutrophil count.
Histological analysis
Colonic segments were fixed in 10 % neutral buffered formalin, embedded in paraffin, sliced into 5 μm-thick sections and then stained using haematoxylin and eosin for histological examination by light microscopy (Nikon). Histological damage was calculated on a ten-point scale as described previously with slight modifications( Reference Dieleman, Palmen and Akol 21 ) (Table 2), and it is expressed as a value for six to eight randomly selected tissue sections from each colon.
Table 2 Histological scores given to haematoxylin and eosin-stained colonic sections to quantify inflammation

Myeloperoxidase activity assay
Colonic segments were homogenised on ice in five volumes of normal saline, and MPO activity was determined using a chemical method as per the manufacturer's instructions (Nanjing Jiancheng Corporation). The degradation of 1 μmol H2O2/min at 37oC is defined as one unit of MPO activity, and the value is expressed as units/g.
Quantitative real-time PCR
Total RNA was extracted from colonic segments using TRIzol (Invitrogen) following the manufacturer's instructions. Reverse transcription of mRNA was carried out using the KeyGene reverse-transcription enzyme according to the manufacturer's protocol. Quantitative real-time PCR was carried out using an ABI 7300 real-time PCR system with a cDNA sample, and amplification was carried out in a 20 μl reaction volume containing 1 × SYBR Green PCR Master Mix (Applied Biosystems) with primers specific for TNF-α (forward: TGAACTTCGGGGTGATCGGTC; reverse: AGCCTTGTCCCTTGAAGAGGAAC)( Reference Wu, Yin and Ernest 22 ), Dectin-1 (forward: GGAATCCTGTGCTTTGTGGTAGTAG; reverse: GGAAGGCAAGACTGAGAAAAACCTC)( Reference Menzies, Henriquez and Alexander 23 ) and β-actin (forward: CCTGAACCCTAAGGCCAACC; reverse: CAGCTGTGGTGGTGAAGCTG)( Reference Gonzales and Orlando 24 ). Relative expression in comparison with that of β-actin was calculated using the comparative computed tomography method.
Statistical analysis
All data are expressed as means with their standard errors. Statistical analysis was carried out using the SPSS 13.0 software (SPSS Inc.), and differences between the groups were analysed using one-way ANOVA followed by Tukey's post hoc test. P< 0·05 was considered to be statistically significant.
Results
Effect of Salecan on food and water intake
The intake of food and water of the mice were monitored daily during the development of DSS-induced colitis. Daily average food intake was not significantly different between the three DSS-treated groups, and relative to the control group, these three groups exhibited decreased food intake (approximately 30 %, P< 0·05; Table 3). There was no significant difference in water intake between the DSS and HS+DSS groups, whereas the LS+DSS group consumed more water.
Table 3 Effect of Salecan on food and water intake (g/d per mouse) during dextran sulphate sodium (DSS) treatment. (Mean values with their standard errors; n 6)
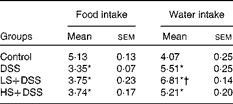
LS, diet with 4 % Salecan; HS, diet with 8 % Salecan.
* Mean value was significantly different from that of the control group (P< 0·05).
† Mean value was significantly different from that of the DSS group (P< 0·05).
Effect of Salecan on the disease activity index
After 5 d of exposure to 4 % DSS in drinking-water, the DSS group developed the clinical symptoms of acute colitis, with weight loss, diarrhoea and rectal bleeding (Fig. 2). Change in weight (>3 %) became prominent after 3 d of DSS treatment (Fig. 2(a)). The presence of blood in the faeces was detected 1 d after the start of DSS treatment, whereas gross bleeding and diarrhoea were initially observed from day 4 onwards (Fig. 2(b) and (c)). As a consequence of the inflammatory process, the DSS group had a clearly worse median disease activity index value, combined score of weight loss, stool consistency and faecal occult blood divided by 3, compared with the control group from day 1 to the end of DSS treatment (Fig. 2(d)). On day 5, mice fed the diet supplemented with 8 % Salecan exhibited markedly reduced disease severity compared with the DSS group, which might be attributed to substantial reductions in stool consistency and faecal occult blood brought about by Salecan (Fig. 2).

Fig. 2 Effect of Salecan on change in weight (a), stool consistency (b), faecal occult blood (c) and disease activity index (d) during dextran sulphate sodium (DSS) treatment. LS, diet with 4 % Salecan; HS, diet with 8 % Salecan. Values are means (n 6 mice per group), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). † Mean value was significantly different from that of the DSS group (P< 0·05). ‡ Mean value was significantly different from that of the LS+DSS group (P< 0·05). ![]() , Control;
, Control; ![]() , DSS;
, DSS; ![]() , LS+DSS;
, LS+DSS; ![]() , HS+DSS.
, HS+DSS.
Effect of Salecan on haematological parameters
Anaemia is a common complication of UC, resulting from mucosal blood loss. As shown in Table 4, the DSS group had severe anaemia (decreased erythrocyte, Hb and haematocrit levels) in comparison with the control group. Supplementation of the diet with Salecan obviously attenuated these symptoms. No evident differences in mean corpuscular volume, mean corpuscular Hb and mean corpuscular Hb concentration were found among these four groups. The neutrophil count of the DSS group was significantly (4-fold) higher than that of the control group, despite similar levels of leucocytes and lymphocytes. The groups treated with Salecan exhibited a lower increase in neutrophil count.
Table 4 Haematological parameters in mouse groups (Mean values with their standard errors; n 6)
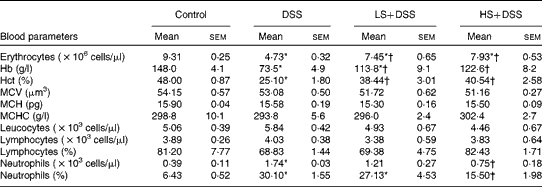
DSS, dextran sulphate sodium; LS, diet with 4 % Salecan; HS, diet with 8 % Salecan; Hct, haematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular Hb; MCHC, mean corpuscular Hb concentration.
* Mean value was significantly different from that of the control group (P< 0·05).
† Mean value was significantly different from that of the DSS group (P< 0·05).
Effect of Salecan on colon length and spleen weight
Colon length is used as a morphometric measure of the degree of colonic inflammation. We observed a conspicuous reduction in the colon length of the DSS group amounting to approximately one-fourth of that of the control group (Fig. 3(a)). The Salecan diet prevented the DSS-induced colon shortening, and a significant effect was observed in the high-dose group. As spleen enlargement is a characteristic of a systemic inflammatory reaction such as that occurring in acute DSS colitis, spleens were also weighed. There was an obvious increase (approximately 30 %) in spleen weight in the DSS group relative to the control group (Fig. 3(b)). However, Salecan did not seem to have a significant effect on the reduction of spleen enlargement.

Fig. 3 Effect of Salecan on the total colon length (a) and spleen weight (b) after dextran sulphate sodium (DSS) administration. LS, diet with 4 % Salecan; HS, diet with 8 % Salecan. Values are means (n 6 mice per group), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). † Mean value was significantly different from that of the DSS group (P< 0·05).
Effect of Salecan on the histological features of dextran sulphate sodium-induced colitis
Histological assessment of the colonic sections of mice treated with DSS for 5 d revealed crypt shortening and loss, ulceration and dense acute inflammation consisting mostly of neutrophils (Fig. 4(a)–(d)). By contrast, colon architecture of the HS+DSS group appeared to be relatively normal, showing only mild evidence of crypt distortion and inflammation with neutrophil infiltration (Fig. 4(e)–(h)). In Fig. 4(i), the histological damage scores of all groups are shown, which clearly demonstrate the ability of Salecan to alleviate tissue destruction and neutrophil infiltration, characteristics of DSS-induced colitis.

Fig. 4 Histological presentation of colitis in dextran sulphate sodium (DSS)-treated mice and protective effects of Salecan. (a, b) Normal histology of the colon in the control group. LS, diet with 4 % Salecan; HS, diet with 8 % Salecan. (c, d) Extensive intestinal ulceration with severe inflammatory cell infiltrate in the DSS group. Amelioration of the inflammatory process and accumulation of goblet cells in (e, f) the LS+DSS group and (g, h) the HS+DSS group. (a), (c), (e) and (g) 100 × magnification; (b), (d), (f) and (h) 400 × magnification. (i) Histological damage scores. Values are means (n 3 mice per group), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). † Mean value was significantly different from that of the DSS group (P< 0·05).
Effect of Salecan on myeloperoxidase activity
Neutrophil accumulation was also confirmed by the measurement of MPO activity. MPO activity was dramatically increased in the colonic tissue samples of the DSS group (Fig. 5). This increase in MPO activity induced by DSS was inhibited by Salecan.

Fig. 5 Effect of Salecan on myeloperoxidase (MPO) activity in mice treated with dextran sulphate sodium (DSS). LS, diet with 4 % Salecan; HS, diet with 8 % Salecan. Values are means (n 6 mice per group) with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). † Mean value was significantly different from that of the DSS group (P< 0·05).
Effect of Salecan on the mRNA levels of TNF-α and Dectin-1
Quantitative real-time PCR were carried out to test the effect of Salecan diet on TNF-α mRNA levels. In contrast to the control group, a substantial up-regulation of colonic TNF-α mRNA levels was observed in the DSS group (Fig. 6(a)). Treatment of experimental colitis with 8 % Salecan resulted in a significant reduction in colonic TNF-α mRNA levels.

Fig. 6 Effect of Salecan on the relative mRNA levels of TNF-α (a) and Dectin-1 (b) in the colon of mice treated with dextran sulphate sodium (DSS). LS, diet with 4 % Salecan; HS, diet with 8 % Salecan. Values are means (n 6 mice per group) with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). † Mean value was significantly different from that of the DSS group (P< 0·05).
As Dectin-1 is implicated in the regulation of cytokine expression in response to β-glucans, we further investigated the mRNA levels of this receptor. As reflected by quantitative real-time PCR, Dectin-1 mRNA levels of the DSS-treated mice were similar to those of the control mice and, as expected, the Salecan diet led to an obvious increase in Dectin-1 mRNA levels, and this increase was associated with an increase in Salecan concentrations (Fig. 6(b)).
Discussion
New drugs and therapy options are urgently needed to prevent and/or cure UC. There is increased interest in the prophylactic and/or therapeutic efficacy of several types of dietary fibres for the treatment of experimental and human UC( Reference Fernandez-Banares, Hinojosa and Sanchez-Lombrana 7 , Reference Kanauchi, Suga and Tochihara 9 , Reference Naito, Takagi and Katada 25 ). Our interest was to evaluate the effects of Salecan as a potential dietary therapeutic compound for UC, which stemmed from its identification as a bioactive β-glucan without toxicity( Reference Zhou, Pu and Xia 17 ) and from the results reported by Lavi et al. ( Reference Lavi, Levinson and Peri 26 ). In the present study, we demonstrated Salecan to attenuate DSS-induced clinical symptoms as well as colonic injury and inflammation in mice. Importantly, we demonstrated Salecan to inhibit the increase in TNF-α mRNA levels caused by DSS treatment and to increase the mRNA levels of the β-glucan receptor Dectin-1.
DSS is a synthesised sulphated dextran with epithelial toxicity, and the DSS model of experimental colitis established in 1990 by Okayasu et al. ( Reference Okayasu, Hatakeyama and Yamada 27 , Reference Solomon, Mansor and Mallon 28 ) is well accepted due to its similarities to human UC in aetiology, pathology, pathogenesis and therapeutic response. Besides, based on the fact that genetic susceptibility and specific immunity do not seem to play important roles in the inflammatory process induced by DSS, this model is one of the most suitable ones to study the environmental components of UC, especially the diet( Reference Lara-Villoslada, Debras and Nieto 29 ). In the present study, mice were given 4 % DSS dissolved in drinking-water for 5 d in an attempt to assess the protective effects of Salecan as a particular component of the diet on acute inflammation. Salecan, especially at high doses, protected the mice from the toxicity of DSS, evidenced both diagnostically, with alleviation of diarrhoea and bloody faeces, amelioration of anaemia, and prevention of histological damage, and biochemically, by a decrease in colonic MPO activity. It should be noted that water intake in the LS+DSS group was higher than that in the DSS group, which meant that the LS+DSS group consumed more DSS (approximately 1362 mg/mouse v. approximately 1102 mg/mouse). Based on the above, one would assume that the LS+DSS group would exhibit a greater degree of colonic damage, which may mask the full impact of the treatment.
Anaemia is a common haematological complication of IBD, occurring in 6 to 74 % of the patients, and negatively affects patients' quality of life( Reference Bergamaschi, Di Sabatino and Albertini 30 ). In accordance with the previous observations made by Larrosa et al. ( Reference Larrosa, Yañéz-Gascón and Selma 31 ), mice treated with DSS had anaemia marked by lower levels of erythrocytes, Hb and haematocrit. This normocytic (with normal mean corpuscular volume) and normochromic (with normal mean corpuscular Hb concentration and mean corpuscular Hb) anaemia is usually associated with inflammation and occurs due to the loss of blood in the faeces. Neutrophils have been suggested to be involved in the development of colitis( Reference Lampinen, Sangfelt and Taha 32 ). In the present study, there were significant increases not only in blood neutrophil counts but also in the number of neutrophils infiltrating the colon, as assessed by the measurement of colonic MPO activity and confirmed by histology, in the DSS group. MPO activity, an index of tissue-associated neutrophil infiltration, is widely used to quantify intestinal inflammation, and a reduction in the activity of this enzyme can be interpreted as a manifestation of the anti-inflammatory activity of a given compound( Reference Camuesco, Gálvez and Nieto 33 ).
The protective effects of dietary Salecan supplementation in DSS-induced experimental colitis can be explained by an anti-inflammatory mechanism through the inhibition of the production and/or release of pro-inflammatory mediators including cytokines. Pro-inflammatory cytokines are known to induce or facilitate inflammation in intestinal mucosa( Reference Nakamura, Saito and Kasanuki 34 ). Specifically, a key role for TNF-α as a pro-inflammatory cytokine in the activation and perpetuation of the inflammatory response in patients with IBD has emerged, and treatment with medications that inhibit TNF-α has been proposed as a new therapeutic strategy( Reference Sandborn and Hanauer 35 ). Infliximab, a chimaeric monoclonal antibody directed against TNF-α, has been approved by the US Food and Drug Administration for the treatment of patients with IBD in 1998( Reference Ordás, Mould and Feagan 36 ). In the present study, the Salecan-supplemented diet exerted a conspicuous attenuating effect on the DSS-induced increase in TNF-α mRNA levels. Such an inhibitory effect of Salecan on TNF-α mRNA levels is in agreement with the results of studies on partially hydrolysed guar gum( Reference Naito, Takagi and Katada 25 ) and on glucans from edible mushroom( Reference Lavi, Levinson and Peri 26 ). Recent studies have started to shed light on Dectin-1. Dectin-1, a non-classical C-type lectin-binding β-1,3-linked glucan, can induce its own intracellular signalling and play a crucial role in the mediation of a variety of inflammatory responses, such as cytokine production( Reference Brown 37 ). Dectin-1 activates and regulates the transcription factor NF-κB through a Syk kinase-dependent signalling pathway and/or a Raf-1 serine–threonine kinase-dependent signalling pathway( Reference Rogers, Slack and Edwards 38 , Reference Gringhuis, den Dunnen and Litjens 39 ). Besides, some studies have demonstrated that Dectin-1 also collaborates with other MyD88-coupled Toll-like receptors in NF-κB signalling, which may be responsible for the synergistic induction of multiple cytokines including TNF( Reference Reid, Gow and Brown 40 ). Shah et al. ( Reference Shah, Williams and Keshvara 41 ) reported that particulate β-glucans suppress cytokine production in response to Toll-like receptor stimulation and that this response is mediated by Dectin-1. Given the facts mentioned above, Dectin-1 mRNA levels were determined by quantitative real-time PCR in the present study. Salecan prominently up-regulated Dectin-1 mRNA levels in a dose-dependent manner, which indicates that the protective effects of Salecan against DSS-induced colitis by the suppression of TNF-α mRNA levels might thus be related to its effect on the up-regulation of Dectin-1 mRNA levels. It should be stressed that, to the best of our knowledge, Dectin-1 has been mentioned for the first time in the study of the effect of β-glucans on DSS-induced colitis.
The treatment of IBD is a burgeoning field. Infliximab, which just appeared a decade ago, has been the most significant addition to the spectrum of therapeutic options( Reference Kozuch and Hanauer 42 ). However, optimal therapy for IBD has not been established still. It has been described that some nutritional supplements are protective against UC, including chitin nanofibrils( Reference Azuma, Osaki and Wakuda 43 ), olive oil( Reference Camuesco, Gálvez and Nieto 33 ), resveratrol( Reference Larrosa, Yañéz-Gascón and Selma 31 ) and probiotic bacteria( Reference Herias, Koninkx and Vos 44 ). The present results suggest that Salecan has potential as a nutritional supplement for patients with UC.
In conclusion, our findings support that Salecan supplementation facilitates the recovery of the inflamed colon in the DSS model of mouse colitis, an effect associated with inhibition of the production of pro-inflammatory mediator TNF-α. This beneficial effect could be ascribed to the enhanced expression of Dectin-1. More developmental research is necessary before Salecan could be used as a functional food for UC patients.
Acknowledgements
The present study was supported by a grant obtained from the 973 Programs (2012CB517505 and 2013CB945203) and JSSTP (BE2011836).
The authors' contributions are as follows: M. Z., Z. W. and J. Z. conceived and designed the research; M. Z., Z. W. and J. C. carried out the experiment; M. Z., Y. Z., T. W. and L. X. carried out the data analysis; M. Z., Z. W., S. W., Z. H. and J. Z. wrote and revised the manuscript; J. Z. had primary responsibility for the final content. All authors read and approved the final version of the manuscript.
None of the authors has any conflicts of interest to declare.














