Type 2 diabetes results from the interplay of genetic(Reference Rich, Norris and Rotter1, Reference van Hoek, Dehghan and Witteman2) and environmental factors(Reference Lyssenko, Jonsson and Almgren3, Reference Meigs, Shrader and Sullivan4), which influence the dysregulation of a number of pathways(Reference Heald, Cade and Cruickshank5–Reference Boden and Laakso8). Differences in the prevalence and incidence of type 2 diabetes between migrant and non-migrant populations may provide important information on the role of environmental factors such as dietary composition(Reference Harding, Day and Khaw9, Reference Mensink, Aro and Den Hond10) and physical activity(Reference Fischbacher, Hunt and Alexander11) in this disease while partially controlling for the effects of some genetic factors(Reference Mbanya, Cruickshank and Forrester12). Type 2 diabetes results in multiple microvascular and macrovascular complications that are often fatal and affect a disproportionate number of persons of African origin in developed countries. Additionally, macrovascular complications from diabetes are the leading cause of death among black populations in developing countries(Reference Lee, Huxley and Lam13, Reference Magliano, Söderberg and Zimmet14). The identification of modifiable dietary risk factors for diabetes in the African Diaspora along with evidence from studies on lifestyle modification(Reference Mayer-Davis, Sparks and Hirst15–Reference Pedersen and Gaede17) should help to reduce the burden of this disease worldwide.
Previously reported findings from the present study population indicated that the habitual diet in rural Cameroon contains more fat and alcohol than the diet in urban Cameroon(Reference Jackson, Walker and Cruickshank18, Reference Mennen, Jackson and Sharma19). Higher levels of physical activity in the rural subsistence farming community may explain the lower levels of obesity despite higher energy consumption. In addition to the differences in dietary patterns, there is variation in rates of glucose intolerance in these populations. Our earlier report described a gradation in the prevalence of diabetes in men and women with the lowest rates in rural and urban Cameroon (0·8 and 2·0 %, respectively), Jamaica having an intermediate prevalence (8·5 %) and African-Caribbeans living in the Manchester, UK, having the highest rates (14·6 %), with the latter two sites additionally having more previously identified cases(Reference Mbanya, Cruickshank and Forrester12).
The present study examines whether intakes of carbohydrate, protein and dietary fat assessed from carefully developed, culture-specific quantitative FFQ (QFFQ) are associated with glucose intolerance in a representative sample of people of African origin from urban Cameroon, rural Cameroon, Jamaica and Caribbean (mainly Jamaican) migrants living in inner-city Manchester, UK(Reference Mennen, Jackson and Sharma19–Reference Mennen, Jackson and Cade23).
Experimental methods
Study population
The present study was part of an international collaboration that assessed nutritional intakes, prevalence of diabetes, hypertension and their risk factors in African origin populations(Reference Mbanya, Cruickshank and Forrester12, Reference Cooper, Rotimi and Ataman24, Reference Cooper, Rotimi and Kaufman25). Samples were recruited from Cameroon (urban and rural), Jamaica (Spanish Town) and the United Kingdom (Manchester). In Cameroon and Jamaica, communities ranging in size from 10 000 to 40 000 adults were sampled according to the ‘probability proportionate to size’ method with equal proportions of men and women in four age categories (25–34, 35–44, 45–54 and 55–74 years). Random samples stratified by age decade and sex were taken from population registers in inner-city Manchester where most African-Caribbeans live. Further details on the sampling scheme have been previously described(Reference Mennen, Jackson and Sharma19, Reference Cruickshank, Mbanya and Wilks26, Reference Cruickshank, Mbanya and Wilks27). Sampling methods, especially in Jamaica, were co-standardised with the International Collaborative Study on Hypertension in Blacks study, which ran contiguously with this, and all sites shared blood pressure measurement standardisation(Reference Cooper, Rotimi and Ataman24, Reference Cooper, Rotimi and Kaufman25, Reference Luke, Durazo-Arvizu and Rotimi28).
Eligibility criteria
People of African origin were recruited if they met at least two of the following criteria: (a) ancestry – at least three grandparents should be of west African origin, (b) observed ethnicity – black African origin – a subjective assessment by the interviewer and (c) self-assigned ethnicity – black African origin. Pregnant women were excluded. The response rates in urban and rural Cameroon were 95 and 98 %, respectively, 62 % in Jamaica and 80 % in the UK. In the UK group, about 73 % were of Jamaican origin and the remainder from other eastern Caribbean territories. The study was approved by national and local ethics committees.
Procedures
The methodology for the cross-site standardisation of procedures and for determining glucose intolerance has been previously published(Reference Mbanya, Cruickshank and Forrester12). A 75 g oral glucose tolerance test was performed on each subject. The 2006 WHO criteria were used to classify subjects according to their glucose tolerance status (normal: fasting plasma glucose < 6·l mmol/l and a 2 h plasma glucose (2 h postprandial glucose) < 7·8 mmol/l; impaired glucose regulation including impaired fasting glucose (IFG) (fasting plasma glucose ≥ 6·1 mmol/l and < 7·0 mmol/l) and impaired glucose tolerance (IGT) (2 h plasma glucose ≥ 7·8 mmol/l but < 11·0 mmol/l); diabetes: fasting plasma glucose ≥ 7·0 mmol/l or 2 h postprandial glucose ≥ 11·1 mmol/l). Sixty-four subjects from all sites reported that they were on treatment for diabetes and were excluded from further analyses. Information on demographic and socioeconomic factors and smoking was obtained by questionnaire. The variables used here were age, education level, marital status, smoking and employment status.
Dietary assessment
The strategy for the development of a dietary assessment tool used to determine food and nutrient intakes has been reported previously(Reference Jackson, Walker and Cade21, Reference Sharma, Cade and Jackson29–Reference Sharma, Yacavone and Cao31). In each country, an interviewer-administered QFFQ was developed to assess habitual nutrient intake during the previous 12 months and included those foods contributing at least 90 % of total energy, fat, carbohydrate and protein intakes. Interviewers used local utensils, especially prepared wooden food models, and cutlery to help subjects to describe their own portion size.
For Cameroon, the questionnaire included seventy-six food items, and the variation in consumption of foods between the wet and dry seasons was ascertained. The Jamaican questionnaire included seventy foods and drinks. The UK questionnaire included 108 food items from the Caribbean as well as from Europe. To calculate the nutrient composition of the habitual diet, several food tables(Reference Tan, Wenlock and Buss32–Reference Holland, Welch and Unwin34) and the nutritional analysis package, Microdiet(Reference Fletcher35), were used. Since these tables did not cover all the typical dishes eaten in Cameroon and the UK, dishes were prepared by local people, and all the contents and the final cooked dish were weighed by trained field workers to determine the nutrient composition of the dishes(Reference Sharma, Cade and Landman20).
The UK questionnaire was calibrated against 24 h recalls and 4 d weighed intake in a subsample of the total population. Each ‘calibrating’ method measures different short-term time periods to the habitual estimate from the QFFQ. Spearman rank correlation coefficients ranged from 0·38 for protein intake to 0·62 for carbohydrate intake when compared with the 4 d weighed intake and from 0·38 for fat intake to 0·50 for energy intake when compared with the 24 h recall. When the QFFQ was compared with the 4 d weighed record, the diet of 39 % of participants fell in identical quartiles and 44 % were in adjacent quartiles, and no participants were grossly misclassified, showing reasonable comparison of these different methods. In Jamaica, the QFFQ responses were compared with twelve 24 h recalls conducted throughout the study period. Correlation coefficients (Pearson and intra-class) varied between 0·42 for retinol and 0·71 for carbohydrate, with most values falling between 0·50 and 0·60. The reproducibility of the QFFQ was tested in a subsample of 118 subjects, and Pearson's correlation coefficients between protein, fat and carbohydrate intakes from the first and the second measurement were 0·62, 0·67 and 0·69, respectively(Reference Jackson, Walker and Cade21).
Statistical methods
The data were analysed using Stata version 10.2 (StataCorp, College Station, TX, USA). An a priori exclusion criterion was used for total energy intakes. Participants with energy intakes more extreme than 2 sd above or below the mean total energy were therefore excluded. The final sample included n 991 (urban Cameroon), n 658 (rural Cameroon), n 847 (Jamaica) and n 246 (UK). As diabetes rates in both Cameroonian settings were low, data from rural and urban Cameroon were combined for some analyses in this report. Anthropometric and metabolic data are expressed as arithmetic means with 95 % CI. Initially, comparisons were made across the three population groups using Kruskal–Wallis, χ2 and ANOVA tests.
Nutrient intakes were adjusted for total energy by computing residuals from regression analyses, with energy intake as the independent variable and nutrient intake as the dependent variable(Reference Mbanya, Cruickshank and Forrester12, Reference Willett, Howe and Kushi36). Residuals were added to the expected nutrient value for the mean energy intake of the sample to obtain a score adjusted for the average energy intake. Variance components models, with fasting glucose and 2 h postprandial glucose as outcome variables, were developed to estimate the intra-cluster correlation and to determine relevance of linear mixed models for these analyses. With each of the four sites regarded as a cluster, random effects generalised linear mixed models (with the identity link) were used to determine the nature of the relationship between the respective nutrient scores and each of fasting glucose and 2 h postprandial plasma glucose levels. The same methodology was used to determine whether the country of origin was an effect modifier of the respective relationships. The results presented are country-specific and cross-country estimates of the increment in the respective outcome variables in response to 0·1 unit increments in the PUFA:SFA (P:S) ratio or 1 unit increments in percentage energy from the respective nutrients. The estimates were adjusted for age, sex, BMI, country of origin, level of education attained, smoking status and tertiles of alcohol consumed.
Mixed effects logistic regression models (generalised linear mixed models with the logit link) yielded OR adjusted for age, sex, country, BMI, level of education attained, smoking status and tertiles of alcohol consumed for the association of glucose tolerance status – IFG/IGT or newly detected type 2 diabetes – with previously mentioned incremental changes in the respective nutrient scores. The models resulted in cross-country and country-specific estimates of the relative odds of having either IGT/IFG or type 2 diabetes v. being normoglycaemic. The models that provided country-specific estimates also indicated whether there was an interaction of country with nutrient intakes in the effect of the latter on the odds of IGT/IFG or type 2 diabetes. As not all participants had all measurements due to missing data, numbers varied between analyses.
Results
Men and women were youngest in Cameroon and oldest in Manchester, UK (Table 1). BMI was highest in the UK for both sexes and lowest in Cameroon, while waist:hip ratios were highest in Manchester men. Overall, there was a cross-site gradient of obesity (BMI ≥ 30 kg/m2) prevalence (Cameroon 16·1 %; Jamaica 31·2 % and UK 35·1 %; χ2 59·1, P = 0·0001); however, male obesity rates were lowest in Jamaica and Cameroon and nearly four times higher in the UK population (UK 20·4 %; χ2 24·5, P = 0·0001). Smoking prevalence was highest in men and women from Manchester. Fasting plasma glucose levels were lowest in Cameroonian participants (Table 1).
Table 1 Anthropometric, demographic and metabolic features of participants by country*
(Mean values and 95 % confidence intervals or percentages)

IGR, impaired glucose regulation for impaired fasting glucose/impaired glucose tolerance combined.
* Comparisons were made using ANOVA or Kruskal–Wallis test for continuous variables or χ2 test for categorical variables.
Table 2 presents the details of energy intake and the percentage contribution of macronutrients by country. Crude-reported total energy intake was highest in Cameroon men and women and lowest in the UK. Percentage energy from total fat was about 10 % higher in Cameroon (mainly from staple palm nut oil) than either Jamaican or UK men or women. However, Jamaicans had the lowest percentage energy from PUFA and SFA. P:S ratios in women were significantly higher in participants from Manchester but were similar in Cameroon and Jamaica.
Table 2 Age-adjusted energy intakes and percentage contribution of macronutrient intakes by country*
(Mean values and 95 % confidence intervals)

% E, energy percentage; P:S, PUFA:SFA.
* Comparisons were made using ANOVA for continuous variables.
Additionally, for both men and women, Table 2 indicates that percentage energy from protein in the UK and Jamaica was significantly higher (P = 0·0001) than in Cameroon. More than 58 % of the reported total energy intake came from carbohydrate in Jamaicans; this was only slightly less at about 52–55 % of total energy in Manchester and less than 50 % of total energy in Cameroon. Daily intake of dietary fibre was significantly higher in Cameroon compared to the other populations.
Relationship between nutrient intake and diabetes status
To account for correlation between responses within each of the four sites, mixed effects logistic regression models were used to obtain OR for the association of different nutrients with occurrence of IFG/IGT and type 2 diabetes. The OR for the association of different energy-adjusted nutrients with the presence of IFG/IGT and type 2 diabetes are shown in Table 3. The results indicate, for all sites combined, that a 1 % increment in energy from protein, total fat and saturated fats significantly increased the odds of type 2 diabetes by 10, 4 and 15 %, respectively. Conversely, each 1 % increment in energy from carbohydrates and a 0·1 unit increment in P:S ratio significantly reduced the odds of having type 2 diabetes by 4 and 12 %. When these crude estimates were adjusted for age, sex, BMI and country (Cameroon, Jamaica and UK), the significant association of percentage energy from protein, carbohydrate, total fat and saturated fat was retained. In separate models adjusting for smoking, the level of educational attainment and tertiles of alcohol consumption did not modify the nature of the respective relationships between the nutrients and the outcomes as shown in Table 3. We found no evidence of an association between IFG/IGT with any of the nutrients.
Table 3 Unadjusted and adjusted country-specific point estimates of the relative odds for impaired fasting glucose/impaired glucose tolerance (IFG/IGT) or type 2 diabetes associated with incremental changes in nutrient intakes as produced by generalised linear mixed models for categorical outcomes
(Odds ratio and 95 % confidence intervals)
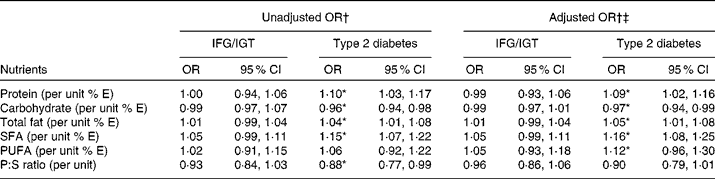
% E, energy percentage; P:S, PUFA:SFA.
* P < 0·05.
† Reference category was normoglycaemia.
‡ Estimates adjusted for age, sex, country and BMI.
Relationship between nutrient intake and diabetes status by site
Mixed effects logistic regression analyses were also used to assess whether the relationship between nutrient intakes and the odds of glucose tolerance status was modified by country. Data presented in Table 4 suggest that country of origin interacts with percentage energy from carbohydrate in its effect on glucose tolerance state. As such, higher percentage energy from carbohydrates significantly reduced the odds of IFG/IGT in persons from Cameroon. After adjusting for age, sex and BMI, higher values of percentage energy from protein, total fat and saturated fat significantly increased the odds of type 2 diabetes in Jamaicans. In a separate model, the independent relationship between the percentage energy from protein or fats and the odds of having type 2 diabetes was not modified when smoking status or tertiles of alcohol consumption were added to the multivariate models. Increasing percentage energy from PUFA significantly increased the odds for type 2 diabetes in the Cameroonian participants. A higher P:S ratio was associated with reduced odds of type 2 diabetes in Jamaicans only.
Table 4 Unadjusted and adjusted country-specific point estimates of the relative odds of impaired fasting glucose and/or impaired glucose tolerance (IFG/IGT) or type 2 diabetes associated with incremental changes in nutrient intakes as produced by generalised linear mixed models for categorical outcomes
(Odds ratios and 95 % confidence intervals)

T2DM, type 2 diabetes mellitus; % E, energy percentage; P:S, PUFA:SFA.
* P < 0·05.
† Estimates adjusted for age, sex and BMI.
Relationship between nutrient intake and glucose as a continuous variable
Generalised linear and latent mixed models (with the identity link function) were used to determine the nature of the relationship between percentage energy from different macronutrients, P:S ratio and glucose levels. There was a positive association between percentage energy intakes from protein and SFA with both fasting glucose and 2 h glucose (Table 5). Unadjusted P:S ratio was borderline associated with fasting glucose (P = 0·05). In addition, a statistically significant negative relationship of the P:S ratio with fasting glucose (P = 0·017) and 2 h postprandial glucose (P = 0·001) was found in both the unadjusted and adjusted analyses (Table 5). The results shown in Table 5 were unchanged by adjustment for each of education, tertiles of alcohol consumption and smoking status.
Table 5 Unadjusted and adjusted country-specific point estimates of the increment in glucose levels (per 1 mmol/l) associated with incremental changes in nutrient intakes as produced by generalised linear mixed models for continuous outcomes
(β Coefficients and 95 % confidence intervals)

% E, energy percentage; P:S, PUFA:SFA.
* P < 0·05.
† Estimates adjusted for age, sex, country and BMI.
‡ Nutrients are per unit percentage energy (per unit % E).
Relationship between nutrient intake and glucose as a continuous variable by country
Table 6 illustrates the results of models aimed at assessing whether country of origin (nationality) modified the relationship between reported nutrient intake and glucose levels. Adjusted results demonstrated a significant direct association between percentage energy from protein and fasting glucose in Jamaicans (0·07 mmol/l higher in participants whose percentage energy from protein is higher by 1 %) and participants from the UK but not in Cameroon. For the Jamaican participants, each unit increase in SFA was associated with a 0·12 mmol/l increase in fasting glucose (0·22 mmol/l for 2 h glucose). There was an inverse relationship between percentage energy from carbohydrates (β = − 0·023) and fasting glucose. The percentage energy from total fat, as a 0·045 mmol/l increase for a 1 unit change in this nutrient index, was also observed with 2 h glucose but not with fasting glucose.
Table 6 Unadjusted and adjusted country-specific point estimates of the increment in glucose level (fasting and 2 h) associated with incremental changes in nutrient intakes as produced by generalised linear mixed models for continuous outcomes stratified by country
(β Coefficients and 95 % confidence intervals)

% E, energy percentage; P:S, PUFA:SFA.
* P < 0·05.
† Estimates adjusted for age, sex, country and BMI.
Discussion
In the present large multisite cross-sectional study of African origin population samples, we found a strong independent association between increasing protein and total fat intakes and the relative risk of type 2 diabetes across all sites. In both linear and logistic regression models, there was a weaker but significant inverse association between increasing P:S ratio and type 2 diabetes as well as fasting and 2 h postprandial glucose, for all sites combined. These results emerged despite complex interactions of factors at each site.
As expected, BMI, age and geographic location (compared with Cameroon) were independently associated with increased odds of IFG/IGT and type 2 diabetes. Although BMI was lower in Jamaican men and similar in Jamaican women compared with Cameroon, the levels of 2 h postprandial glucose and rates of type 2 diabetes were significantly higher, indicating that factors other than adiposity are important in determining glucose handling. Protein and carbohydrate intakes as percentage of total energy were higher in both Jamaica and the UK, which also had higher levels of 2 h glucose and rates of type 2 diabetes.
While studies in non-diabetic subjects have linked high fat intakes, primarily a high intake of saturated fat (SFA), with an increased risk of CHD(Reference Keys37–Reference Anderson, Sanders and Cruickshank45), the epidemiological evidence linking dietary fat with type 2 diabetes has been inconsistent. Dietary fat is, however, of particular interest because fatty acids influence glucose metabolism by altering cell membrane function, enzyme activity, insulin signalling and gene expression(Reference Risérus, Willett and Hu46). High intakes of SFA may induce insulin resistance and thus worsen glycaemic control(Reference Louheranta, Schwab and Sarkkinen47). Improvements in glycaemia as well as dyslipidaemia have been found with increased consumption of low glycaemic index foods in metabolic studies(Reference Liu and Willett48). In populations, generally at higher risk of insulin resistance, such as the US population, it is suggested that diets with increased levels of unsaturated fatty acids, particularly MUFA, have been suggested to have several advantages over high-carbohydrate intakes and may lower the prevalence of diabetes(Reference Grundy, Abate and Chandalia49). In contrast, a recent European study of carbohydrate intake and the incidence of type 2 diabetes found that a higher carbohydrate intake at the expense of protein and PUFA might be linked to a reduction in diabetes risk(Reference Schulze, Schulz and Heidemann50).
Data from the Iowa Women's Health Study have suggested that the composition of dietary fat intake may play a role in the development of diabetes with an inverse relationship between fat subtype and type 2 diabetes(Reference Meyer, Kushi and Jacobs51). Self-reported dietary intakes generally suggest an inverse association between n-6 PUFA intake and diabetes risk(Reference Hu, van Dam and Liu52). Furthermore, as we found here, the European Prospective Investigation of Cancer–Norfolk study reported that a higher energy-adjusted dietary polyunsaturated:saturated fat ratio (P:S ratio) was associated with a reduced risk of type 2 diabetes, independent of age, sex, family history of diabetes and other lifestyle factors(Reference Harding, Day and Khaw9). The present findings are broadly in agreement with this. Taken together, the evidence from published studies suggests that replacing saturated fats and trans-fatty acids with unsaturated (polyunsaturated and/or monounsaturated) fats has beneficial effects on insulin sensitivity and is likely to reduce the risk of type 2 diabetes(Reference Risérus, Willett and Hu46). Among polyunsaturated fats, linoleic acid from the n-6 series has been shown to improve insulin sensitivity. This contrasts with data from clinical trials of low-carbohydrate diets that contain a greater proportion of saturated fat, yet resulting in improvement of glucose and lipid control even among persons with type 2 diabetes(Reference Summers, Fielding and Bradshaw53, Reference O' Neill, Westman and Bernstein54).
Previously reported results from the present study population have indicated that the habitual diet in rural Cameroon contains more fat and alcohol than the diet in urban Cameroon. Much higher levels of physical activity in the rural subsistence farming community together with lower BMI may explain the lower levels of the cardiovascular risk factors in this area compared to those of the urban dwellers(Reference Mennen, Mbanya and Cade22). Parallel data show that energy balance rather than diets high in fat per se is a primary cause of excess body fat in Western society(Reference Willett and Leibel42).
Differences in the association between nutrient intakes and dysglycaemia may be a reflection of the habitual dietary profiles for the different populations investigated here(Reference Sharma, Cade and Jackson29). Foods containing carbohydrates, primarily from rice and peas, bread, green bananas and mangoes, were important contributors to reported energy intake in Jamaica. Cassava contributed 44 % of the carbohydrate energy intake in rural Cameroon. The inverse relationship observed between carbohydrate and glucose levels in the Jamaican participants may be a consequence of the proportion of dietary fibre(Reference Liu, Manson and Stampfer55), fruits(Reference Liu, Manson and Lee56), vitamins and antioxidants, a marker of a healthy diet and a negatively correlated glycaemic index(Reference Liu, Willett and Stampfer57). In contrast, fats primarily from animal sources, in Jamaica but more so in the UK adults, adversely affect glucose metabolism, and are reported to increase diabetes incidence, independent of obesity and lifestyle factors(Reference Meyer, Kushi and Jacobs51). Nuts (rich in fibre and Mg) contributed 5 and 12 % of energy from saturated fat and polyunsaturated fat to the higher reported energy intake in the Cameroonian respondents (who had the lowest rate of dysglycaemia), supporting the results of a meta-analysis of prospective studies which showed that an increased consumption of Mg-rich foods such as whole grains, beans, nuts and green leafy vegetables may reduce the risk of type 2 diabetes(Reference Larsson and Wolk58).
The mechanisms mediating the effect of dietary macronutrients on insulin resistance and glucose handling are complex. It may be the case that protein intake serves as a marker for foods which are diabetogenic. It has been argued that while protein does not contribute to sustained elevations of glucose levels nor slow absorption of carbohydrate, it is just as potent a stimulant of insulin secretion as glucose(Reference Franz59). A higher protein intake may be a marker of a more affluent lifestyle with attendant lower activity levels.
The findings of the present study accord with those of the Nurses' Health Study, which demonstrated an increase in the risk of diabetes with increasing red and other processed meat intake during 14 years of follow-up(Reference Fung, Schulze and Manson60). A similar association was also demonstrated in the Women's Health Study where the significantly increased diabetes risk appeared to be most pronounced for frequent consumption of total processed meat(Reference Song, Manson and Buring61). These results remained significant after further adjustment for intakes of dietary fibre, glycaemic load and total fat. More recently, the European Prospective Investigation into Cancer and Nutrition study demonstrated that diabetes risk increased with higher total protein and animal protein intakes at the expense of percentage energy from carbohydrates or fat(Reference Sluijs, Beulens and van der62).
Study limitations
This is a cross-sectional study and, as such, is subject to a number of biases or confounding factors. As with all cross-sectional studies, the temporal ordering among the relationships observed cannot be established. Physical activity, an important determinant of energy expenditure, was not measured in the present study. Another potential limitation of the present study is the lack of adjustment for the effects of lifetime exposure to urban environment and recent migration or current residence, which have been identified as risk factors for obesity and diabetes mellitus(Reference Sobngwi, Mbanya and Unwin63). Estimates of dietary intakes are obtained from QFFQ that have the potential for measurement error, and respondents are known to under-report dietary intakes (although energy intakes reported here remained high). It is possible that there are other confounders that were not examined in the present study that could also lead to residual confounding.
Conclusions
Diet energy contributed by specific macronutrients may contribute to IFG/IGT and diabetes prevalence between and within African origin population sites. In multivariate analyses, each unit increase in energy from protein and fats was associated with a increased risk of type 2 diabetes, while an increased P:S ratio was associated with a reduction in risk. These independent effects of dietary factors, within genetically similar groups at different stages of the nutrition transition, are further evidence for modifiable lifestyle effects on impaired glucose handling. The study highlights the potential for specific nutritional components of diabetes prevention programmes in at-risk populations worldwide.
Acknowledgements
S. G. A. and N. Y. participated in the study analysis, interpretation of data, drafting of the manuscript and provided statistical expertise. M. K. T.-R., A. H. H. and W. P. S. participated in the interpretation of data and critical revision of the manuscript. J. K. C., R. W., T. F., B. B., J.-C. M., M. D. J., A.T. and S.S. participated in the study concept and design, acquisition of data, interpretation of data and critical revision of the manuscript. All the authors provided input to the manuscript, and none of the authors had any conflict of financial or personal interest. S. G. A. is currently an NIHR Academic Clinical Fellow in Cardiovascular Medicine and was supported as a medical student by a BNF Travel Award and a Denis Burkit Travel Award from the British Nutrition Foundation. The present study was supported by a grant from the European Commission no. TS3*CT92-0142. We would like to thank the Project Staff and all respondents for their participation in the present study.










