Diabetes is a disease characterised by a hyperglycaemic state that leads to a chronic inflammatory status( Reference King 1 ). In addition, co-morbidities such as hypertension, dyslipidaemia, central obesity and insulin resistance that act simultaneously with hyperglycaemia have been involved in the exacerbation of inflammatory status in diabetic patients( Reference Goldberg 2 ). Chronic inflammation plays a major role in the pathogenesis of its secondary complications such as atherothrombosis, nephropathy, retinopathy, CVD and insulin resistance in these patients( Reference King 1 – Reference Spranger, Kroke and Möhlig 3 ).
Diet is the first line of intervention for preventing and treating diabetes, and its complications such as inflammation. In recent years, there has been growing interest in the role of different dietary determinants in the modulation of inflammation. Diets rich in anti-inflammatory components such as fibre, Mg and phenolic compounds constitute a promising preventive strategy against increasing inflammation( Reference Weglarz, Wawszczyk and Orchel 4 – Reference Nilsson, Johansson and Ekström 8 ). These components reduce inflammation by decreasing lipid oxidation and hyperglycaemia, and by down-regulating genes involved in the inflammatory pathway( Reference Chacko, Song and Nathan 9 – Reference González, Ballester and López-Posadas 11 ). Moreover, a diet with a low glycaemic index has been reported to be inversely associated with inflammatory marker levels( Reference Griffith, Ma and Chasan-Taber 12 ). Foods with a high glycaemic index have been shown to induce hyperglycaemia and hyperinsulinaemia that may lead to the production of advanced glycation end products, which may stimulate the liver to increase the production of acute-phase reactants( Reference Liu, Manson and Buring 13 ).
Legumes have a low glycaemic index, and also contain high contents of anti-inflammatory nutrients such as fibre, Mg and phenolic compounds( Reference Bouchenak and Lamri-Senhadji 14 ). Previous cross-sectional studies have reported that dietary patterns with higher intakes of legumes are inversely associated with high-sensitivity C-reactive protein (hs-CRP)( Reference Lopez-Garcia, Schulze and Fung 15 , Reference Esmaillzadeh, Kimiagar and Mehrabi 16 ). However, some clinical trials evaluating the effect of dietary legumes on inflammation have documented inconsistent results; inclusion of legumes in the diet has been reported to modulate the levels of inflammatory markers among obese subjects( Reference Hermsdorff, Zulet and Abete 17 ) and subjects at risk for colorectal cancer( Reference Hartman, Albert and Zhang 18 ), but not in hypercholesterolaemic subjects or patients with peripheral artery disease( Reference Zahradka, Wright and Weighell 19 , Reference Winham and Hutchins 20 ).
Although many clinical trial studies have indicated that intake of soyabean, being a subgroup of legumes, reduces the levels of inflammatory markers( Reference Azadbakht, Kimiagar and Mehrabi 21 , Reference Nasca, Zhou and Welty 22 ), the components of soyabean differ from those of non-soya legumes. The isoflavone and protein contents of soyabean are very high compared with those of non-soya legumes. Furthermore, compared with most non-soya legumes that are very low in fat (generally containing < 5 % of energy), soyabean contains 47 % fat( Reference Messina 23 ). The high content of fibre and complex carbohydrate and the low glycaemic index of non-soya legumes differ from those of soyabeans( Reference Messina 23 ). Thus, while soyabeans are efficacious in the reduction of inflammatory markers, their mode of action may be very distinct from that of non-soya legumes.
Therefore, considering the scarcity and inconsistency of the evidence available for the effect of non-soya legumes on inflammatory markers among diabetic patients, the present study aimed to determine the effect of non-soya legume inclusion in the therapeutic lifestyle change (TLC) diet on inflammatory markers among type 2 diabetic patients.
Materials and methods
Participants
The present randomised cross-over clinical trial aimed to compare the effects of two intervention diets (legume-free TLC diet and non-soya legume-based TLC diet) among type 2 diabetic patients. Of the participants who attended the clinic of Taleghani hospital, Tehran, Iran, during 2012, a total of 157 diabetic patients were screened. Inclusion criteria were as follows: age between 50 and 75 years; having a clinical diagnosis of type 2 diabetes (fasting blood glucose concentrations ≥ 7 mmol/l ( ≥ 126 mg/dl) or using oral glucose-lowering medications and having a stabilised status for the past 3 months); being non-smokers; BMI 25–30 kg/m2; not currently receiving insulin therapy; not having any impairments of cardiac, hepatic or renal function. Of the 157 diabetic patients, 115 were excluded due to lacking inclusion criteria (n 61) and refusal to participate (n 54). After exclusion, forty-two patients were eventually assigned to a 2-week run-in period on a usual diet (50 % of energy from carbohydrate, 15 % of energy from protein and 35 % of energy from fat, with restrictions that included no type of legume), with no changes in lifestyle during this period. At the end of the run-in period, two of the participants refused to participate. The remaining forty patients were randomly allocated to intervention groups by an assistant using random sequencing generated in SPSS. Of these remaining participants, half (n 20) were assigned to the legume-free TLC diet and the other half (n 20) to the non-soya legume-based TLC diet. The intervention period was followed by a washout period of 4 weeks, after which the groups followed the alternate treatment for 8 weeks. Of these forty participants, seven dropped out due to poor compliance with the treatment protocol and two other due to change in medications. Therefore, a final group of thirty-one patients completed the two intervention diets( Reference Hosseinpour-Niazi, Mirmiran and Hedayati 24 ). Because the present study was a dietary intervention, the patients and investigators were not blinded. Participants were requested not to change their habitual physical activity levels during the study, and to document their physical activity and 3 d dietary records, including 2 weekdays and 1 weekend day, at baseline and during the intervention for each week, to be reviewed by the study staff for checking dietary compliance and assessing nutrient intakes. The present study was approved by the Research Council and the Ethics Committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving patients were approved by the Iranian Registry of Clinical Trials (identification no. IRCT201202251640N7). Written informed consent was obtained from all patients.
Diets
For each patient, two dietary interventions were prescribed: (1) legume-free TLC diet and (2) non-soya legume-based TLC diet. The legume-free TLC diet included a macronutrient composition of 50–60 % of energy from carbohydrate, 15 % of energy from protein and 25 to 35 % of energy from fat ( < 7 % saturated fat, up to 20 % monounsaturated fat and up to 10 % polyunsaturated fat), as well as intake of < 200 mg cholesterol and 25–30 g fibre. The non-soya legume-based TLC diet was the same as the control diet, except that two servings of red meat were replaced by different types of cooked legumes such as lentils, chickpeas, peas and beans for 3 d per week; half a cup of cooked legumes was considered as one serving of red meat. Patients were not on a weight-reducing diet because weight loss was not the goal of the present study. Laboratory staff were blinded to the treatment status of the participants. For the assessment of dietary compliance, each participant was visited every week for 30 to 45 min at baseline and during the intervention. During each weekly visit, compliance was assessed using the 3 d dietary records. Participants were educated by dietitians on the substitution of red meat by legumes, and advised to reduce SFA and cholesterol intakes and increase fibre intakes. Participants also received education on using an exchange list of foods and preparation of the dietary record. Each participant had to bring his/her 3 d dietary record, including 2 weekdays and 1 weekend day, at each visit. Each food and beverage was then coded according to the prescribed protocol and analysed for the content of energy and the other nutrients using the Nutritionist III software (version 7.0; N-Squared Computing), exclusively designed for Iranian foods. Estimated nutrient intakes from all records collected during the intervention were averaged to determine dietary compliance. In the legume-free TLC diet, mean intakes of energy, protein, fat, SFA, MUFA and carbohydrate were determined from the dietary record and divided by the recommended amounts of these nutrients according to energy requirements of each patient, based on equations suggested by the Institute of Medicine, and Food and Nutrition Board( 25 ). Compliance was found to be >90 %. Moreover, intakes of cholesterol and fibre were adjusted according to the TLC diet ( < 200 mg cholesterol and 25–30 g fibre intake). In the non-soya legume-based TLC diet, in addition to the aforementioned data, intakes of legumes >90 % (of those prescribed) were considered as good compliance.
Measurements
Fasting venous blood samples were taken after an overnight fast of 12–14 h at baseline and after 8 weeks of dietary intervention. On the day of blood collection, serum was separated and frozen at − 70°C for biochemical analysis. Serum hs-CRP concentration (pg/ml) was measured using the ELISA kit (Diagnostics Biochem Canada, Inc.). IL-6 (pg/ml) and TNF-α (pg/ml) concentrations were also measured using the ELISA kit (Diaclone). The intra-assay CV of all assays were < 5 %. The sensitivity of assays for hs-CRP, IL-6 and TNF-α was 0·2 mg/l, 2 pg/ml and 2 pg/ml, respectively.
Statistical analysis
Statistical analysis was performed using SPSS for Windows version 15.0 (SPSS). Initially, normal distribution of all variables was examined by the Kolmogorov–Smirnov test. For the non-normal distributions of hs-CRP, TNF-α and IL-6, logarithmically transformed values of these variables were used in all analyses, and their geometric means are reported. End point and baseline treatment values were used to calculate the change in each variable. General linear models and paired Student's t tests were used to compare the means of inflammatory markers at the end of the two different dietary intervention periods and the mean change for each variable in the two intervention groups. Statistical significance was defined as P< 0·05.
Results
The baseline characteristics of the participants are summarised in Table 1. Mean age and weight of the participants (77·4 % females) who completed the study were 58·1 (sd 6·0) years and 74·5 (sd 7·0) kg, respectively. No significant differences were observed in the baseline characteristics of the patients across the dietary intervention periods. The physical activity level of the legume-free TLC diet group was 2·56 (sd 0·2) metabolic equivalent hours per d and of the non-soya legume-based TLC diet group was 2·49 (sd 0·2) metabolic equivalent hours per d. These levels remained the same across all the study periods. Of the thirty-one participants, twenty-eight used lipid-lowering medications, thirty used blood pressure-lowering medications, and all participants used oral glucose-lowering medications.
Table 1 Baseline characteristics of the study participants (Mean values and standard deviations)
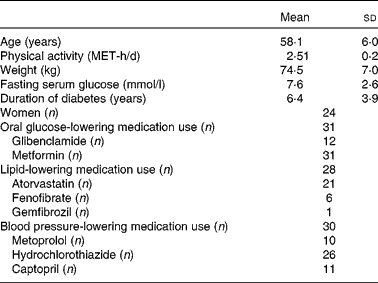
MET-h, metabolic equivalent hours.
Compared with the legume-free TLC diet group, dietary intakes of total fibre (26·9 (se 1·3) v. 31·4 (se 1·5) g, P= 0·03) and Mg (408 (se 4·2) v. 450 (se 5·4) mg, P= 0·03) increased significantly, whereas dietary intakes of Fe (31·9 (se 1·2) v. 17·2 (se 1·3) mg, P= 0·04) and cholesterol (129 (se 9) v. 169 (se 14) mg, P= 0·04), and glycaemic index (49·8 (se 1·8) v. 42 (se 1·3), P= 0·04) decreased significantly in the non-soya legume-based TLC diet group, as revealed by the analysis of the self-reported 3 d dietary record. No significant differences were observed between the legume-free TLC diet group and the non-soya legume-based TLC diet group in relation to total energy intake (8610 (se 112) v. 8493 (se 104) kJ), percentage of protein (14·6 (se 0·7) v. 13·9 (se 0·5)), percentage of total fat (33·9 (se 0·8) v. 32·5 (se 0·6)), percentage of carbohydrate (52·2 (se 1·7) v. 53·6 (se 1·3)), percentage of SFA (8·4 (se 0·5) v. 7·1 (se 0·5)), percentage of MUFA (17·1 (se 0·9) v. 17·5 (se 0·9)) or percentage of PUFA (8·3 (se 0·4) v. 7·9 (se 0·4)).
The effects of dietary interventions on inflammatory markers are outlined in Table 2. After consumption of both the legume-free TLC diet and the non-soya legume-based TLC diet, hs-CRP, TNF-α and IL-6 concentrations decreased significantly from their baseline values. Compared with the legume-free TLC diet, the non-soya legume-based TLC diet significantly decreased the concentrations of hs-CRP ( − 1·3 (se 1·1) v. − 1·7 (se 1·2), P= 0·019), IL-6 ( − 1·2 (se 1·0) v. − 1·6 (se 1·1), P= 0·018) and TNF-α ( − 1·3 (se 1·1) v. − 1·8 (se 1·1), P= 0·018). The weight of the participants did not change significantly after consumption of either the legume-free TLC diet or the non-soya legume-based TLC diet.
Table 2 Concentrations of the inflammatory markers at baseline and after 8 weeks of dietary interventions in type 2 diabetic patients† (Mean values and standard errors)

TLC, therapeutic lifestyle changes; hs-CRP, high-sensitivity C-reactive protein.
Mean value was significantly different from that at baseline: * P< 0·05, ** P< 0·001 (paired Student's t test).
† The control diet was the legume-free TLC diet. The non-soya legume-based TLC diet was the same as the control diet, except that two servings of red meat were replaced with different cooked legumes such as lentils, chickpeas, peas and beans over a period of 3 d per week. End point and baseline treatment values were used to calculate the change in each variable.
Discussion
In the present study, we observed that an 8-week non-soya legume-based TLC diet intervention had favourable effects on inflammatory markers that were associated with type 2 diabetes. This may be explained in part by the comparison of nutrient contents between the two diets: for example, the non-soya legume-based TLC diet had a higher concentration of Mg and fibre, known to be associated with decreased concentrations of inflammatory markers( Reference Song, Li and van Dam 5 , Reference Nilsson, Johansson and Ekström 8 ). In a cross-sectional study, two dietary patterns, one high in Mg and the other (Western dietary pattern) low in Mg, have been reported to be inversely and positively associated with increased inflammatory marker levels and endothelial dysfunction, respectively( Reference Lopez-Garcia, Schulze and Fung 15 ). A high intake of Mg has been reported to be associated with lower concentrations of markers of systemic inflammation and endothelial dysfunction( Reference Chacko, Song and Nathan 9 ), possibly through the down-regulation of genes related to metabolic and inflammatory pathways including C1q and TNF-related protein 9, and pro-platelet basic protein( Reference Chacko, Sul and Song 10 ). Dietary fibre reduces inflammation by decreasing lipid oxidation and hyperglycaemia, which, in turn, are associated with decreased inflammation. Normal bowel flora also contribute to a healthy intestinal environment achieved by dietary fibre intakes, which help to prevent inflammation( Reference King 26 ). Sources of protein intake (i.e. animal or plant) may have different effects on inflammatory markers. In the present study, substitution of animal protein with plant protein may be another component of non-soya legume-based TLC diet that may reduce inflammation. The present result is consistent with that of a previous study demonstrating that substitution of one serving of total red meat intake with an alternative protein food source (e.g. poultry, fish, legumes or nuts) is associated with lower concentrations of C-reactive protein, ferritin, glycosylated Hb and fasting insulin( Reference Ley, Sun and Willett 27 ). In a prospective study, during an 8-year follow-up, vegetable protein consumption has been shown to be inversely associated with endothelial dysfunction and inflammation, whereas no association has been observed between animal protein consumption and these markers( Reference van Bussel, Soedamah-Muthu and Henry 28 ). Moreover, less intake of vegetable protein has been associated with an increase in TNF-α and soluble vascular cell adhesion molecule-1 concentrations( Reference van Bussel, Soedamah-Muthu and Henry 28 ). In addition, in a clinical trial study, after 8 weeks of dietary intervention, higher intakes of animal and meat protein have been reported to be positively associated with inflammatory score based on CRP, IL-6, TNF-α and plasminogen activator concentrations, whereas no association has been found between vegetable protein intake and inflammation in obese individuals with metabolic syndrome symptoms( Reference Lopez-Legarrea, de la Iglesia and Abete 29 ). The mechanisms of the effects of animal and plant protein intakes on inflammatory markers have not been elucidated; however, these effects may be mediated through obesity( Reference Berg and Scherer 30 ). Obesity increases the production of pro-inflammatory cytokines from adipose tissue, and these cytokines induce hepatic production of CRP and other acute-phase proteins( Reference Montonen, Boeing and Fritsche 31 ). However, much remains to be learned about the underlying mechanisms that mediate the multiple effects reported to date. Moreover, lower intakes of cholesterol( Reference Kleemann, Verschuren and van Erk 32 ) and Fe( Reference Wessling-Resnick 33 ) from the non-soya legume-based TLC diet might in part explain these associations.
Cross-sectional and prospective studies have found that consumption of legumes and legume-based diets is inversely associated with type 2 diabetes( Reference Villegas, Gao and Yang 34 , Reference Venn and Mann 35 ). The biological mechanisms underlying these relationships are not entirely understood. One possible mechanism by which legumes has been reported to influence diabetes and it complications is the modulation of inflammation. Serum hs-CRP, an acute-phase protein and a sensitive marker of subclinical inflammation, has been found to be associated with impaired glucose homeostasis, β-cell dysfunction and development of insulin resistance, and therefore diabetes( Reference Nyström 36 – Reference Yuan, Zhou and Tang 38 ). Recent trials have found that adherence to a legume-enriched diet lead to the improvement of the biomarkers of inflammation( Reference Zhang, Monk and Lu 6 ). In an animal model with acute colitis, a high-bean diet has been shown to reduce the mRNA expression of colonic inflammatory cytokines (IL-6, IL-9, interferon-γ and IL-17A), and increase anti-inflammatory IL-10( Reference Zhang, Monk and Lu 6 ). In a cross-over trial, reductions in hs-CRP and soluble TNF-α receptor I levels have been reported among men at risk for colorectal cancer following consumption of a low glycaemic index and a high-legume diet( Reference Hartman, Albert and Zhang 18 ). Adherence to energy-restricted, legume-based diets (four servings/week) for 8 weeks has been shown to result in a significant reduction of hs-CRP, but not IL-6 and TNF-α concentrations, compared with an energy-restricted, legume-free diet among obese participants( Reference Hermsdorff, Zulet and Abete 17 ). In addition, inclusion of barley kernels and brown beans in meals has been shown to decrease inflammatory markers, such as IL-6, by − 17 and − 35 %, respectively( Reference Nilsson, Johansson and Ekström 8 , Reference Nilsson, Ostman and Holst 39 ). Moreover, in a cross-sectional study, plasma concentrations of hs-CRP, TNF-α and IL-6 have been reported to be decreased across increasing quintiles of dietary legumes among healthy women( Reference Esmaillzadeh and Azadbakht 40 ). In contrast, in a clinical trial study, daily consumption of half a cup of cooked legumes (beans, peas, lentils and chickpeas) for 8 weeks has not been shown to change serum markers of endothelial dysfunction and inflammation among twenty-six individuals with peripheral artery disease( Reference Zahradka, Wright and Weighell 19 ). In another study, daily intake of half a cup of baked beans has not been reported to change hs-CRP concentrations among hypercholesterolaemic patients after 8 weeks( Reference Winham and Hutchins 20 ).
Compared with previous studies investigating the effect of legumes in the framework of an energy-restricted diet, the present study found that in an isoenergetic TLC diet, replacement of two servings of red meat with legumes for a period of 3 d per week had beneficial effects on the biomarkers of inflammation in overweight diabetic patients, independent of weight change. The underlying mechanisms by which legume consumption might affect inflammation are not entirely clearly documented. Legumes contain non-digestible fermentable components (SCFA precursors), high fibre such as phytic acid (a major fibre-associated component of legumes), Mg and phenolic compounds (phenolic acids, flavonoids and anthocyanins), all with documented anti-inflammatory potentials( Reference Weglarz, Wawszczyk and Orchel 4 – Reference Nilsson, Johansson and Ekström 8 ). Dietary polyphenols have been found to inhibit cellular enzymes, such as phospholipase A2, cyclo-oxygenase and lipoxygenase, in order to reduce the production of arachidonic acid, PG and leukotrienes, thus exerting an important anti-inflammatory action( Reference Santangelo, Varì and Scazzocchio 41 ). Dietary polyphenols also inhibit NF-κB signalling and the down-regulation of the expression of pro-inflammatory markers( Reference González, Ballester and López-Posadas 11 ). In addition, the beneficial effects of legumes on inflammatory biomarkers might be explained by the low-glycaemic index values of legumes( Reference Qi and Hu 42 ). A high dietary glycaemic index increased the pro-inflammatory process through hyperglycaemia, hyperinsulinaemia and insulin resistance( Reference Liu, Manson and Buring 13 ). Hyperglycaemia and hyperinsulinaemia induced by foods with a high-glycaemic index may lead to the production of advanced glycation end products, which may stimulate the liver to increase the production of acute-phase reactants( Reference Liu, Manson and Buring 13 ).
Our previous investigation has found that compared with the legume-free TLC diet, consumption of the non-soya legume-based TLC diet significantly decreased cardiometabolic risk factors such as fasting blood glucose ( − 19·5 v. − 28·7, P< 0·001), fasting insulin ( − 1·5 v. − 3·5, P= 0·006), TAG ( − 19·5 v. − 38·5, P= 0·02) and LDL-cholesterol ( − 8·7 v. − 15·6, P= 0·02) concentrations( Reference Hosseinpour-Niazi, Mirmiran and Hedayati 24 ). In the present study, a reduction in the inflammatory markers, which was independent of weight change, could have been due to a reduction in the aforementioned cardiometabolic risk factors. Hyperglycaemia, dyslipidaemia and insulin resistance have been known to be involved in the exacerbation of inflammatory status through the up-regulation of their genesis( Reference Eiselein, Wilson and Lamé 43 – Reference Festa, D'Agostino and Howard 48 ).
As regards the limitations of the study, one limitation was that the intervention diets were based on specific dietary recommendations, and pre-prepared diets were not given to the participants; thus, the diets may not have been followed as carefully as in trials where prepared food is provided. In addition, the biochemical index of legume intakes was not measured due to limited funds; however, our findings show that compliance with the non-soya legume-based TLC diet has benefits on inflammatory markers. The non-blinding of patients and investigators due to the nature of the trial (dietary intervention) is another limitation of the present study. Although the rate of non-compliance was also a limitation, with about one-quarter of diabetic patients being lost to follow-up, considering that it was a cross-over trial carried out over a long period of time, a greater number of patient exclusion is common.
Conclusion
Replacement of two servings of red meat with non-soya legumes in the isoenergetic TLC diet for a period of 3 d per week reduced plasma concentrations of hs-CRP, IL-6 and TNF-α among overweight diabetic patients, independent of weight change.
Acknowledgements
The authors thank the participants who took part in the present study. The authors appreciate Ms N. Shiva for critical editing of English grammar and syntax of the manuscript.
The present study was supported by the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran (grant no. 411).
The authors' contributions are as follows: S. H.-N. conceived the study; P. M., S. H.-N. and F. A. designed the study; S. H.-N. and A. F.-G. analysed and interpreted the data; S. H.-N., A. F.-G. and P. M. prepared the manuscript; F. A. supervised the project and approved the final version of the manuscript. All authors read and approved the final manuscript.
There are no conflicts of interest.





