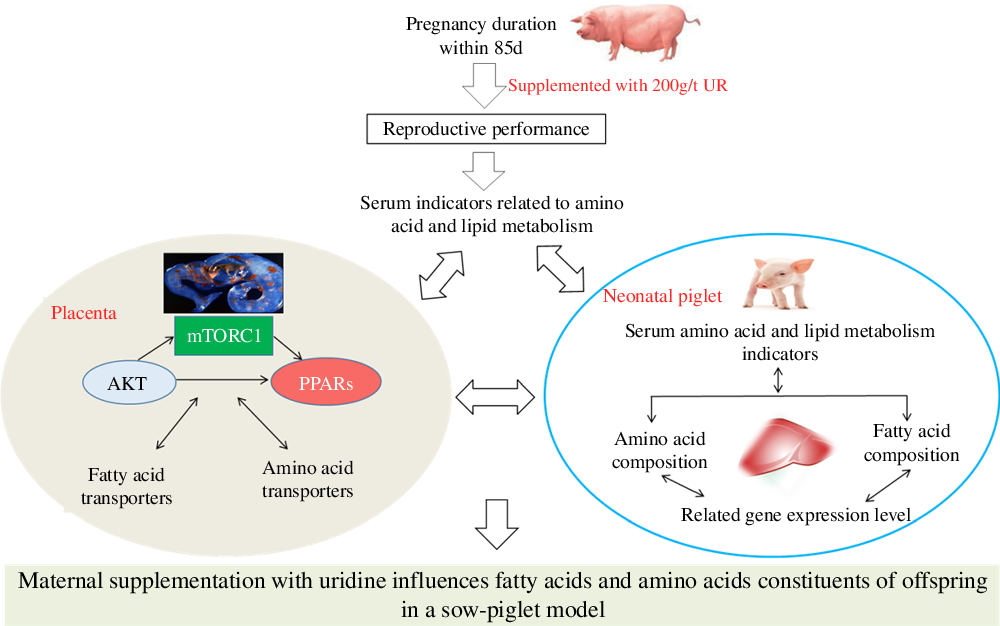
Maternal nutrition status plays a vital role in the growth and development of the major fetal organ systems(Reference Funston and Vonnahme1), and the fetal growth is dramatically increased during late gestation(Reference Campos, Silva and Donzele2). The stillbirth rate is increased with the increasing number of litter farrowed(Reference Vallet, Miles and Brown-Brandl3). Thus, to improve the reproductive efficiency of sows, it is vital to increase the survival rate of newborns during late gestation. The studies showed that fatty acid and amino acid metabolism of sow and fetus affects the development of the fetus, which in turn affects fetal or neonatal survival rate(Reference Cai, Jia and Lu4,Reference Wu, Yin and Liu5) . Therefore, increasing fetal fatty acid and amino acid metabolism levels is the main method to improve reproduction performance of sows.
Nucleotides are abundant in milk(Reference Stein, Peters and Mateo6) and play an essential role in several biological processes, such as enhancing growth(Reference Daneshmand, Kermanshahi and Mesgaran7), improving immunity(Reference Waititu, Yin and Patterson8), enhancing amino acid contents and regulating fatty acid composition in animals(Reference Tie, Wu and Jiang9). The supplementation of nucleotides could provide growth benefits because the synthesis of nucleotides may become limiting during fast growth or stressful conditions(Reference Martinez-Puig, Manzanilla and Morales10). As a kind of nucleotide, uridine monophosphate (UMP) represented 98 % of all the monophosphate nucleotides present in the colostrum of sows(Reference Stein, Peters and Mateo6). Uridine (UR), as a metabolic product of UMP, plays a critical role in regulating energy homoeostasis(Reference Deng, Wang and Gordillo11), protein and lipid metabolism(Reference Liu, Guo and Xie12,Reference Jastroch and Tschöp13) . Several studies showed that UR is very important in regulating the metabolism of amino acid and fatty acid, as well as growth performance. On the one hand, dietary supplementation with UMP or UR during the immediate post-weaning improved the growth performance in weaned piglets or broiler chickens(Reference Xie, Wang and Li14,Reference Li, Xie and Wang15) ; besides, dietary nucleotides can regulate amino acid metabolism in fish(Reference Tie, Wu and Jiang9). UR also plays an important role in regulating energy homoeostasis and bile acid metabolism via regulating lipid metabolism(Reference Deng, Wang and Gordillo11). Short-term UR treatment to early-weaned piglets might stimulate de novo lipid synthesis via the AKT pathways(Reference Zhang, Guo and Xie16), it also affected cholesterol and bile acid metabolism of mice(Reference Zhang, Liu and Zhang17) and the diurnal variations of lipid metabolism in the liver of mice can be regulated by UR(Reference Liu, Zhang and Yin18). Thus, UR plays an important role in the metabolism of amino acids and fatty acids in the sows.
A sow–piglet model was chosen for its physiological, gastrointestinal and blood biochemical indexes that are similar to humans(Reference Shenton, Jie and Uetrecht19); moreover, sows and neonatal piglets are more similar in size to human mothers and infants than other animals(Reference Wu, Xie and Guo20). However, little is known about the direct impact of maternal UR supplementation on the growth and development of offspring. Based on the previous findings, it could be hypothesised that maternal UR supplementation has more beneficial effects on the amino acid and fatty acid metabolism of offspring. Therefore, the current study was conducted to evaluate the effects of maternal dietary UR supplementation during late pregnancy on fatty acid and amino acid constituents and their mechanisms in neonatal piglets.
Material and methods
Ethical statement
Animal experiments were approved by the Animal Care Committee of the Institute of Subtropical Agriculture, Chinese Academy of Science. And, all procedures were performed in accordance with guidelines established by the committee (2015-8A).
Experimental design, animals and management
Fifty-two pregnant sows (Large White × Landrace) at 85 d of gestation with similar parity (3–6 parities), back-fat thickness (approximately 15·04 mm) and close parturition date were randomly assigned into one of the two dietary treatments (CON or UR group with twenty-six sows each). The experiment started once sows had reached day 85 of gestation and continued until the end of farrowing. Experiments were carried out in Henan Guang’an Biology Technology Co. Ltd. Housing and breeding management of experimental animals were as recently described in detail(Reference Gao, Lin and Xie21).
Diets
The single-column feeding was used in this study; the sows in the CON group were fed the basal diet, while sows in the UR group were fed the basal diet supplemented with 200 g/t UR. UR concentration at 200 g/t is the average UMP values of sows’ milk during lactation minus the value of feed of piglets and sows(Reference Li, Zhou and Wu22). UR (purity ≥ 990·0 g/kg) was provided by Meiya Co. Ltd. Sows were fed maize–soyabean-based diets, and all nutrients in the basal diet met the nutrient recommendation of the NRC 2012 for sows (Table 1). All sows were fed twice daily at 06.30 and 15.00 hours and had free access to water during the experiment period. Each sow was fed 2·5–3·0 kg/d from days 85 to 107 of pregnancy and approximately 1·8 kg/d on day 5 before delivery.
Table 1. Composition and nutrient levels of diets
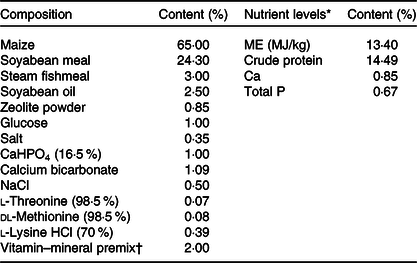
ME, metabolisable energy.
* The nutrient levels were calculated values.
† The vitamin–mineral premix provided the following per kg of diets: sweetening agent 200 mg, antioxidant 100 mg, 2·5 mg of vitamin A, 75 μg of vitamin D3, 15 mg of vitamin E, 1·8 mg of vitamin K3, 2·0 mg of thiamine, 6·0 mg of riboflavin, 4·0 mg of pyridoxine, 0·02 mg of vitamin B12, 26·0 mg of niacin, 18·0 mg of pantothenic acid, 3·2 mg of folic acid, 0·4 mg of biotin, 20 mg of Cu as CuSO4.5H2O, 100 mg of Zn as ZnSO4.H2O, 50 mg of Mn as MnSO4.H2O, 1·2 mg of iodine as KI, 0·30 mg of Se as Na2SeO3. Feed carrier was zeolite powder.
Sample collection
Back-fat thickness was measured at P2 (6 cm from the mid line at the head of the last rib) with an ultrasonic device (Agroscan A16) on days 85 and 114 after pregnancy, and then, the average value was computed.
Farrowing was not induced, and no attempts were made to interfere with the natural delivery of the piglets. Reproductive performance of sows was recorded at delivery, such as the duration of the farrowing and placenta expulsion of sows, litter size, the number of stillbirths, and intra-uterine growth restriction; the number of mummified fetuses (early or middle gestation deaths) was neglected. And within each litter, intra-uterine growth restriction piglets (618–869 g) were defined as weighing approximately 65 % of the birth weight of the largest littermate(Reference Myrie, Mackay and Van Vliet23). After farrowing, the average piglet weight/litter was recorded.
During delivering, 5 ml blood was collected from eight sows per group from the jugular vein with a vacuum blood collection tube. In addition, on the day of birth, eight newborn male piglets (1·50 (sem 0·204) kg) were randomly selected from each of the eight sows marked above as soon as they were born and they were kept apart from sows to avoid ingestion of colostrum(Reference Wu, Gao and Liu24). And then, they were anaesthetised with an iv injection of sodium pentobarbital (50 mg/kg body weight) and bled by exsanguination(Reference Deng, Yao and Chu25). And then, 5 ml of blood was collected aseptically in vacuum blood collection tubes without heparin from the heart. Blood was centrifuged for 15 min at 3000 g under normal temperature to obtain serum and then stored at –80°C until analysis. Moreover, two liver samples were collected from the piglets, one of the livers stored in liquid N2 for quantitative real-time PCR analyses, another liver tissue was stored at –20°C for fatty acid and amino acid analyses.
Allantochorion tissue samples (n 8) in a similar place of placenta of sixteen neonatal boars from sows marked above were obtained immediately during farrowing(Reference Gao, Lin and Xie21). Samples were collected and stored in liquid N2 for real-time PCR and Western blot analyses.
Sample analysis
Serum chemistry
Serum samples were assayed for total protein, aspartate transaminase, glucose (GLU), urea nitrogen, alanine aminotransferase, albumin, alkaline phosphatase, ammonia (NH3), total cholesterol (CHOL), total TAG, HDL, LDL, total bile acids (TBA), lactic acid (LACT) by an Automated Biochemistry Analyzer (Synchron CX Pro, Beckman Coulter), and all the kits were purchased from Beijing Chemlin Biotech Co. Ltd.
Amino acids in serum and liver
Serum (0·5 ml) was deproteinised with 0·5 ml of 1·5 mm HClO4, followed by addition of 0·25 ml of 2 m K2CO3. The neutralised extract was analysed for amino acids using HPLC in Beijing Aminolabs, Co. This method involved the precolumn derivatisation of amino acids with phthaldialdehyde and fluorescence detection. Amino acids in samples were quantified on the basis of known amounts of standards (Sigma Chemicals).
Weighed 0·2 g liver sample that is freeze-dried, crushed, then mixed with 1 ml of 0·1 mol/l HCl and extracted for 30 min, followed by centrifugation for 10 min at 5000 r/min at 4°C. Supernatant was separated and mixed with equal volume of 8 % sulfosalicylic acid, let stand for a night and centrifuged for 5 min at 4°C. Supernatant was separated and filtered to the sample bottle with a 0·45 μm microporous membrane. An L-8800 automatic amino acid analyzer (L8800, Hitachi) was used to determine the concentrations of amino acids in the liver(Reference Wu, Zhang and Liu26).
Medium- and long-chain fatty acids in the liver of neonatal piglets
Lipid from the liver was extracted with chloroform-methanol according to Folch et al., and transmethylated with boron trifluoride (BF3) and methanolic KOH(Reference Morrison and Smith27). Fatty acids were determined by GC (Agilent 6890), and results were expressed as a percentage of total fatty acids.
Quantitative real-time PCR
Allantochorion and liver tissue samples were homogenised under liquid N2, and mRNA was extracted as previously described by Gao et al.(Reference Gao, Lin and Xie21). The relative mRNA expression levels of β-actin (reference gene), the genes related transporters in the placenta and related amino acid and fatty acid metabolism in liver of neonatal piglets were determined by real-time PCR using Luminaris Color HiGreen High ROX (Thermo Scientific) on a Bio-Rad iCycler according to the manufacturer’s instructions. The primers used are shown in Table 2. Fold changes in mRNA expression levels were calculated using the 2–ΔΔCt method.
Table 2. Primers used for real-time PCR
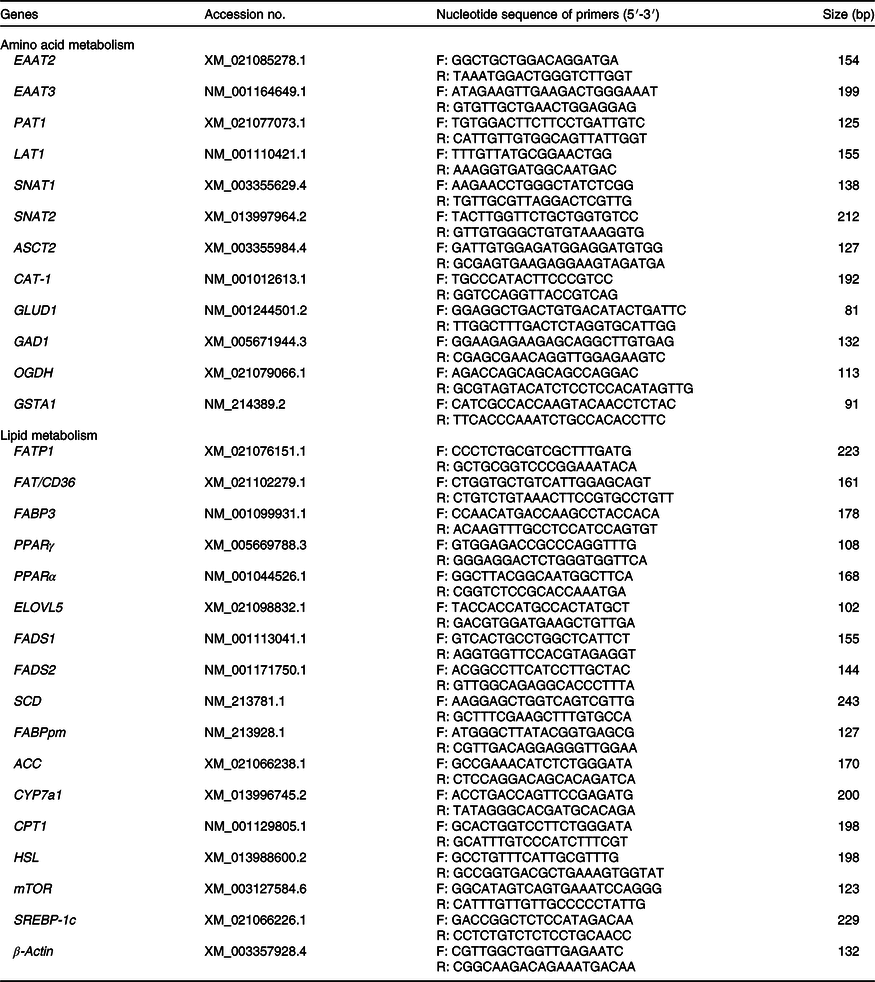
EAAT2, excitatory amino acid transporters 2; F, forward; R, reverse; EAAT3, excitatory amino acid transporters 3; PAT1, proton-coupled amino acid transporter 1; LAT1, neutral amino acid transporter 1; SNAT1, Na-dependent neutral amino acid transporter 1; SNAT2, Na-dependent neutral amino acid transporter 2; ASCT2, amino-acid transporter 2; CAT-1, cationic amino acid transporter 1; GLUD1, glutamate dehydrogenase 1; GAD1, glutamate decarboxylase 1; OGDH, recombinant oxoglutarate dehydrogenase; GSTA1, glutathione S-transferase α1; FATP1, fatty acid transport protein 1; FAT/CD36, fatty acid transporter/CD36; FABP3, fatty acid binding protein 3; ELOVL5, fatty acid elongase 5; FADS1, fatty acid desaturase 1; FADS2, fatty acid desaturase 2; SCD, stearoyl coenzyme A desaturase; FABPpm, fatty acid-binding protein; ACC, acetyl-CoA carboxylase; CYP7a1, cholesterol-7a-hydroxylase; CPT1, carnitine palmitoyl transferase 1; HSL, hormone-sensitive lipase; mTOR, mammalian target of rapamycin; SREBP-1c, sterol regulatory element-binding protein 1c.
Total protein extraction and Western blotting
The total protein extraction of placental tissue was as recently described in detail(Reference Xie, Wu and Long28). Western blotting was performed as described previously(Reference Wu, Xie and Yin29). The membranes were incubated with primary antibodies, including total mTOR (#9461, Cell Signaling Technology), P-mTOR (#5536, Cell Signaling Technology), AKT (#9272, Cell Signaling Technology), phosphorylated protein kinase B (P-AKT; #9271, Cell Signaling Technology), Raptor (#2280, Cell Signaling Technology), PPARα (ab233078, abcam) and PPARγ (ab209350, abcam) at 1:1000 dilution, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (sc-47778) at 1:2000 dilution. At first, the membranes were incubated for 2 h with horseradish peroxidase-linked secondary antibodies (Beijing ZhongShan Golden Bridge Biological Technology Co. Ltd) after being washed with TBST. And then, the membranes were developed by using Super Signal West Dura Extended Duration Substrate according to the manufacturer’s instructions (Pierce) after being washed with TBST. Alpha Imager 2200 (Alpha Innotech Corporation) software was used to quantify Western blots images by measuring the intensity of correctly sized bands, and all protein measurements were normalised to GAPDH.
Statistical analysis
All data were sorted by Excel 2010 after the initial order and analysed using SPSS 21.0 (2015, IBM-SPSS Inc.). All data were expressed as the mean values with their standard errors. Pearson’s correlation between serum biochemical parameters of sows and neonatal piglets was carried out by GraphPad Prism 7.00 for Windows (GraphPad Software). Differences between mean values were compared using independent samples t test and considered significant at P < 0·05. Trends were identified when 0·05 < P < 0·10.
Results
Productive performance
The productive performance of sows is shown in Tables 3 and 4. Compared with the CON group, maternal dietary UR supplementation significantly decreased the birth mortality (P = 0·050) and farrowing and placenta expulsion of sows had a downtrend in the UR group (P = 0·069). There were no differences in the BF of sows, the total born, born alive and intra-uterine growth restriction rate (P > 0·050).
Table 3. Effects of maternal supplementation with uridine (UR) during late pregnancy on the back-fat (BF) thickness of sows
(Mean values with their standard errors; n 26)
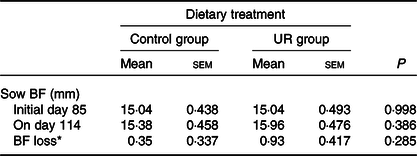
* BF loss = BF of sows on day 114 – BF of sows on day 85.
Table 4. Effects of maternal supplementation with uridine (UR) during late pregnancy on reproductive performance of sows
(Mean values with their standard errors; n 26)

IUGR, intra-uterine growth restriction; FARPLA, farrowing and placenta expulsion of sows.
* Birth mortality = (total litter size – alive)/total × 100.
† IUGR rate = the number of IUGR/total × 100.
‡ FARPLA = Duration of farrowing and placenta expulsion of sows, FARPLA is defined as the time interval between the birth of the first piglet and the expulsion of the last placenta.
Serum biochemical parameters of sows-neonatal piglets
Compared with the CON group, maternal dietary UR supplementation increased total protein, albumin, NH3, TBA and LACT and decreased GLU, CHOL and LDL in the serum of sows (P < 0·05). In addition, serum TAG of sows has observed an uptrend and serum HDL of sows had a downtrend in the UR group (Fig. 1, 0·05 < P < 0·10).
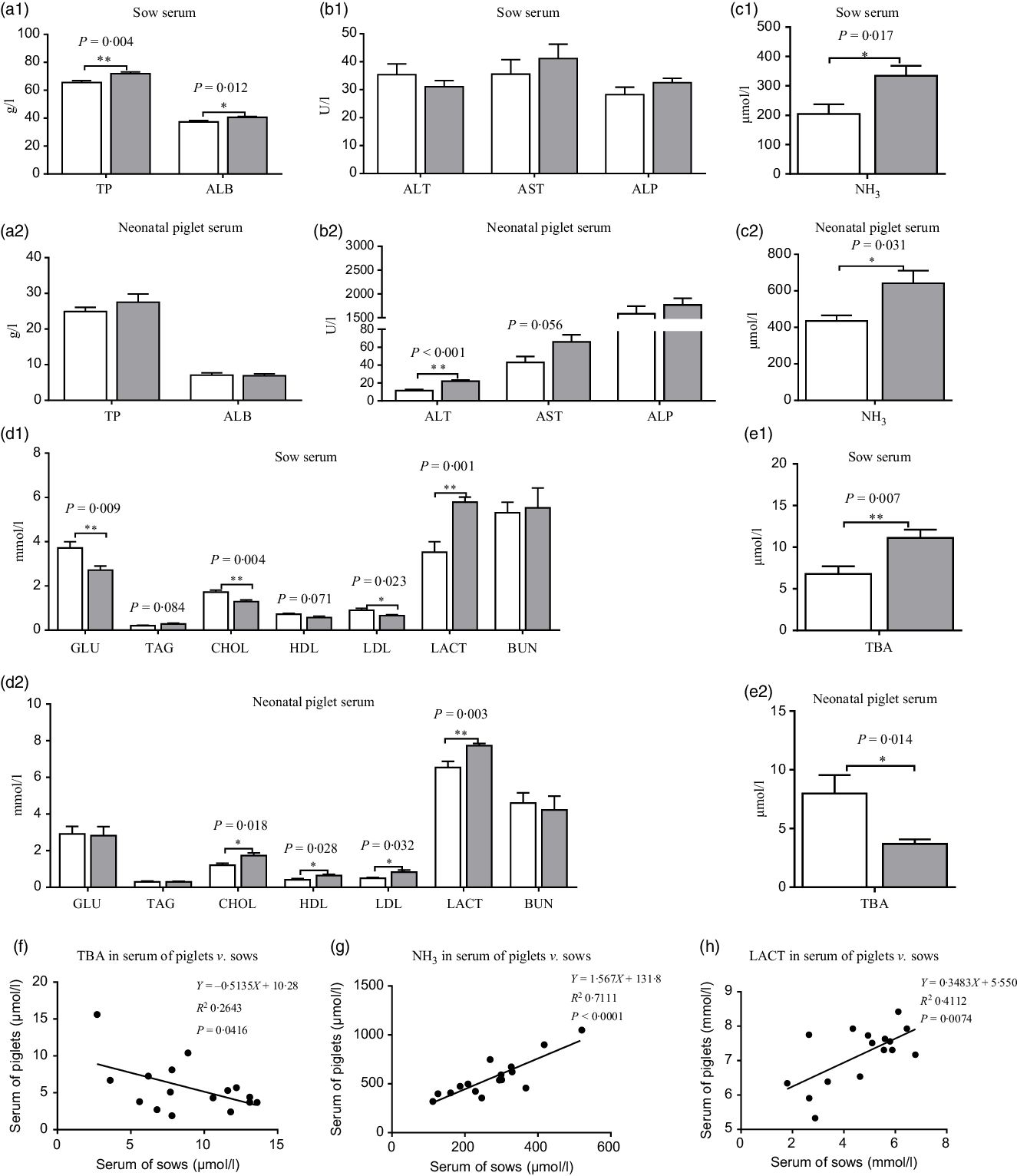
Fig. 1. (a–e) Effects of maternal supplementation with uridine (UR) on serum biochemical parameters of sows and neonatal piglets. Data are mean values with their standard errors, n 8. Statistical significance was set at * P < 0·05 or ** P < 0·01 by t test. (f) Correlation analysis for total bile acids (TBA) in serum of piglets v. sows; (g) correlation analysis for NH3 in serum of piglets v. sows; (h) correlation analysis for lactic acid (LACT) in serum of piglets v. sows. TP, total protein; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate transaminase; ALP, alkaline phosphatase; GLU, glucose; CHOL, total cholesterol; BUN, urea nitrogen. ![]() , Control group;
, Control group; ![]() , UR group.
, UR group.
Moreover, maternal dietary UR supplementation increased alanine aminotransferase, CHOL, LDL, HDL, LACT and NH3 and decreased TBA in the serum of neonatal piglets (P < 0·05). In addition, serum TAG of neonatal piglets had an uptrend in the UR group compared with the CON group (Fig. 1, 0·05 < P < 0·10).
The results of Pearson correlation (r) are presented in Fig. 1, there were different levels of correlation for serum biochemical parameters between sows and neonatal piglets in the two groups (P < 0·05) and, consequently, an association of TBA (r –0·514, P = 0·0416; Fig. 1(f)), NH3 (r 0·843, P < 0·0001; Fig. 1(g)) and LACT (r 0·641, P = 0·007; Fig. 1(h)) was detected in the serum of piglets v. sows.
Amino acid concentrations in the serum of sows and neonatal piglets
The serum amino acid concentrations of sows and neonatal piglets are shown in Table 5. Compared with the CON group, dietary UR supplementation increased tyrosine, threonine, isoleucine, cysteine, methionine, hydroxy-l-proline and β-alanine, and decreased ornithine in the serum of sows. In addition, arginine and phenylalanine in the serum of sows had an uptrend in the UR group (0·05 < P < 0·10).
Table 5. Effects of maternal uridine (UR) supplementation at late pregnancy on serum free amino acid concentrations of sows and neonatal piglets (mg/l)
(Mean values with their standard errors; n 8)
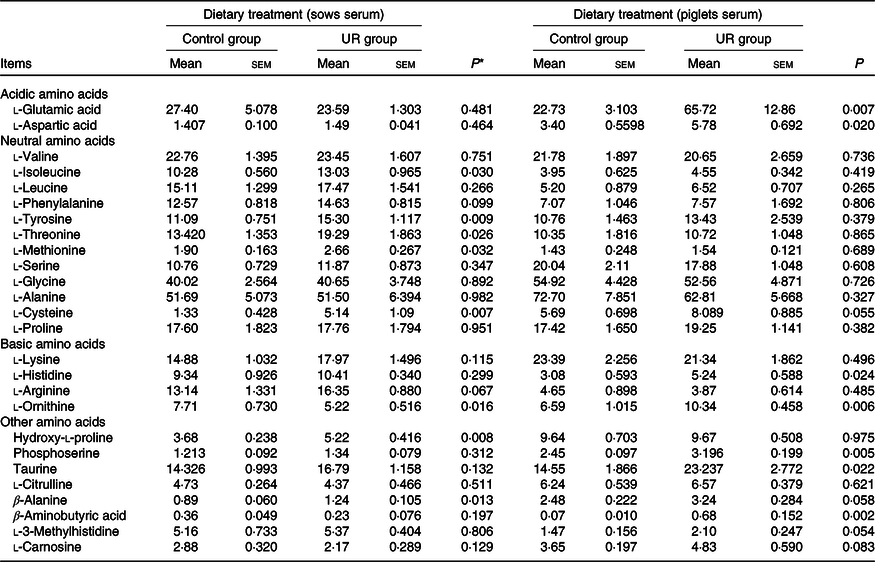
* Probability values < 0·05 and < 0·01 were considered statistically significant and extremely significant, respectively.
Maternal dietary UR supplementation increased glutamic acid, aspartic acid, histidine, ornithine, phosphoserine, taurine and β-aminobutyric acid in the serum of neonatal piglets (P < 0·05). In addition, cysteine, β-alanine, 3-methylhistidine and carnosine in the serum of piglets had an uptrend in the UR group compared with the CON group (Table 5, 0·05 < P < 0·10).
Amino acid and fatty acid constituents in liver of neonatal piglets
Compared with the CON group, maternal dietary UR supplementation increased most amino acids concentrations in the liver of neonatal piglets, including glutamic acid, aspartic acid, lysine, histidine, arginine, valine, isoleucine, leucine, phenylalanine, threonine, serine, methionine, glycine, alanine and proline (Fig. 2, P < 0·05).
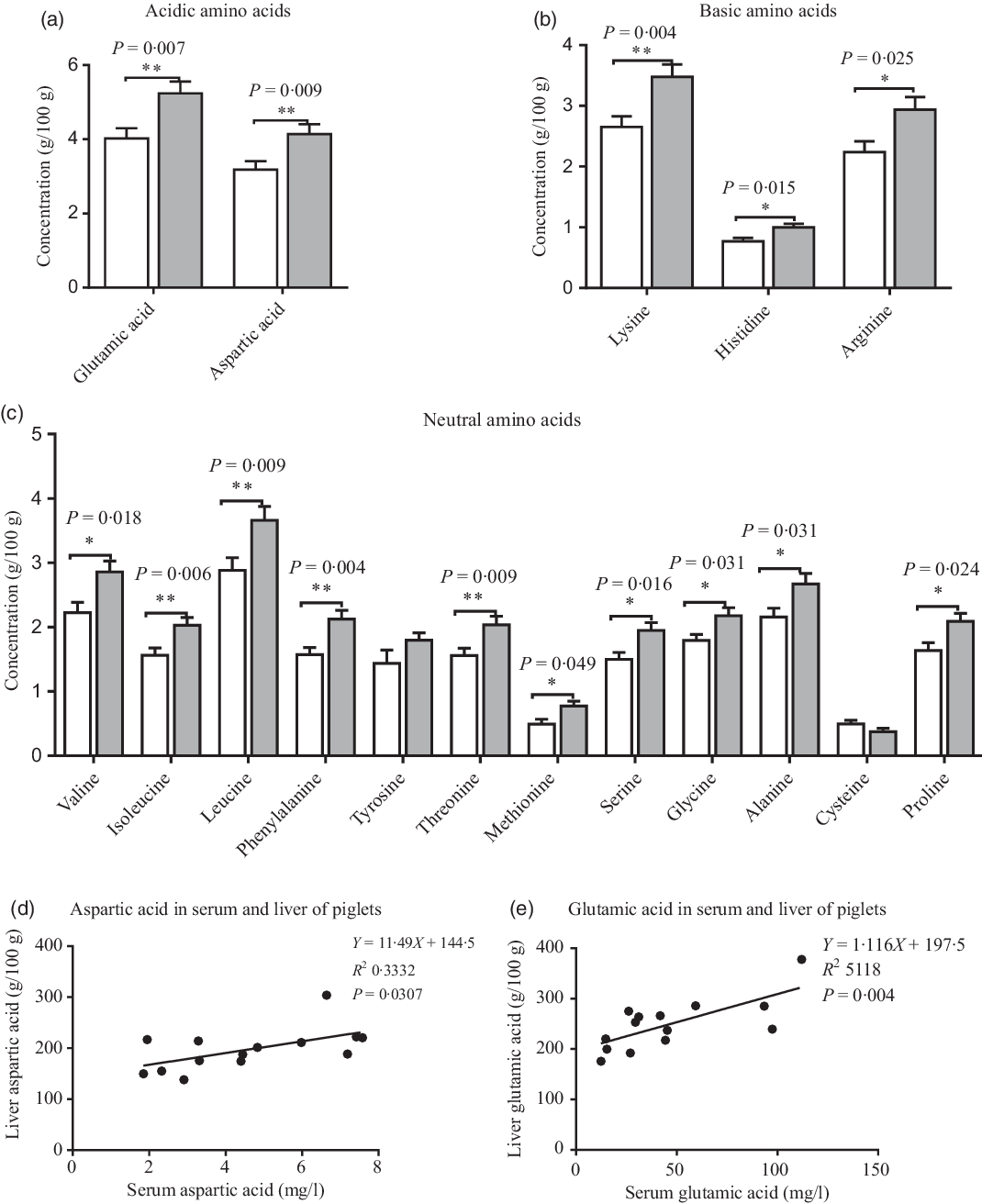
Fig. 2. Effects of maternal uridine (UR) supplementation at late pregnancy on amino acid concentrations in liver of neonatal piglets. Data are mean values with their standard errors, n 8. Statistical significance was set at * P < 0·05 or ** P < 0·01 by t test. ![]() , Control group;
, Control group; ![]() , UR group.
, UR group.
There were different levels of correlation for amino acids in the serum and liver of piglets (P < 0·05). Correlation results of aspartic acid (r 0·577, P = 0·03; Fig. 2(d)) and glutamic acid (r 0·715, P = 0·004; Fig. 2(e)) were recorded in the serum and liver of piglets (Fig. 3).
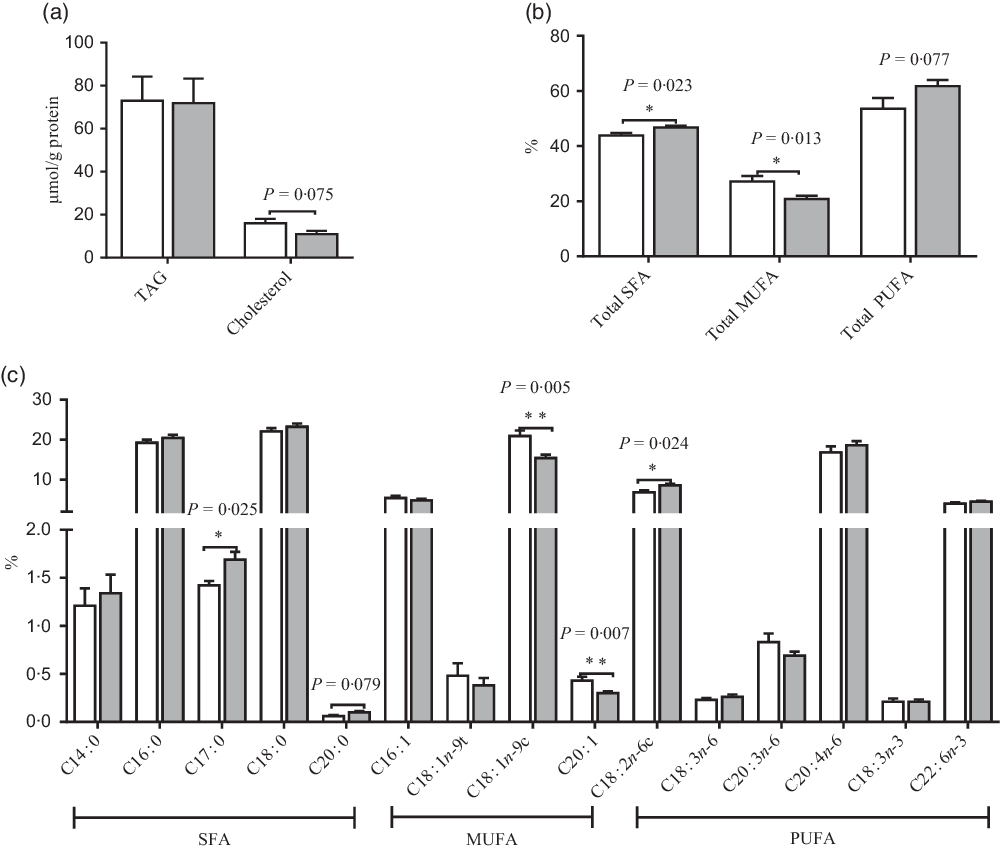
Fig. 3. Effects of maternal uridine (UR) supplementation at late pregnancy on TAG and cholesterol (a) and fatty acids (b and c) in liver of neonatal piglets. Data are mean values with their standard errors, n 8. Total SFA includes C14 : 0, C16 : 0, C17 : 0, C18 : 0 and C20 : 0; total MUFA includes C16 : 1, C18 : 1n-9t, C18 : 1n-9c and C20 : 1; total PUFA includes C18 : 2n-6c, C18 : 3n-6, C20 : 3n-6, C20 : 4n-6, C18 : 3n-3 and C22 : 6n-3. ![]() , Control group;
, Control group; ![]() , UR group.
, UR group.
Compared with the CON group, maternal dietary UR supplementation increased the ratio of C17 : 0, C18 : 2N6C, total SFA, while decreased C18 : 1N9C, C20 : 1 and total PUFA in liver of neonatal piglets (P < 0·05, Fig. 3). In addition, the ratio of C20 : 0 had an uptrend, and cholesterol in the liver of neonatal piglets had a downtrend in the UR group (0·05 < P < 0·10).
Relative gene expression of amino acid and fatty acid metabolism in the liver of neonatal piglets
The mRNA expression of amino acid and fatty acid metabolism in neonatal piglets’ liver is shown in Figs. 4 and 5. Compared with the CON group, maternal dietary UR supplementation increased the mRNA expression of SNAT1, SNAT2, GSTA1, PPARα, FATP1, ELOVL5, FADS1, cholesterol-7a-hydroxylase (CYP7a1) and hormone-sensitive lipase (HSL) in the liver of neonatal piglets (P < 0·05), while decreased the mRNA expression of FABP3, ACC and sterol regulatory-element binding protein 1c (SREBP-1c) in the liver of neonatal piglets (P < 0·05).

Fig. 4. Effects of maternal uridine (UR) supplementation at late pregnancy on mRNA expression of genes involved in amino acid (AA) transport (a) and amino acid metabolism (b) in liver of neonatal piglets. Data are mean values with their standard errors, n 8. Statistical significance was set at * P < 0·05. LAT1, neutral AA transporter 1; PAT1, proton-coupled amino acid transporter 1; SNAT2, sodium-dependent neutral amino acid transporter 2; SNAT1, sodium-dependent neutral amino acid transporter 1; ASCT2, amino acid transporter 2; CAT-1, cationic amino acid transporter 1; EAAT2, excitatory amino acid transporters 2; OGDH, recombinant oxoglutarate dehydrogenase; GAD1, glutamate decarboxylase 1; GLUD1, glutamate dehydrogenase 1; GSTA1, glutathione S-transferase α1. ![]() , Control group;
, Control group; ![]() , UR group.
, UR group.
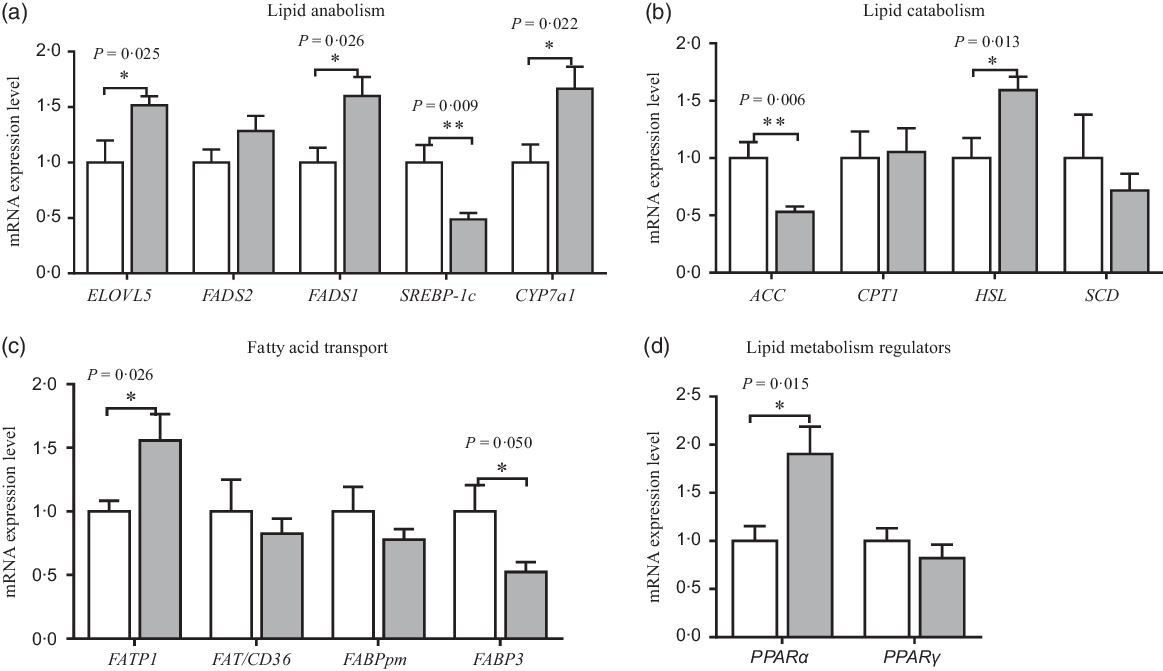
Fig. 5. Effects of maternal uridine (UR) supplementation at late pregnancy on mRNA expression of genes involved in lipid anabolism (a), catabolism (b), transporters (c) and regulators (d) in liver of neonatal piglets. Data are mean values with their standard errors, n 8. Statistical significance was set at * P < 0·05 or ** P < 0·01 by t test. ELOVL5, fatty acid elongase 5; FADS1, fatty acid desaturase 1; FADS2, fatty acid desaturase 2; SREBP-1c, sterol regulatory element-binding protein 1c; CYP7a1, cholesterol-7a-hydroxylase; ACC, acetyl-CoA carboxylase; CPT1, carnitine palmitoyl transferase 1; HSL, hormone-sensitive lipase; SCD, stearoyl coenzyme A desaturase; FATP1, fatty acid transport protein 1; FAT/CD36, fatty acid transporter/CD36; FABPpm, fatty acid-binding protein; FABP3, fatty acid binding protein 3. ![]() , Control group;
, Control group; ![]() , UR group.
, UR group.
Gene and protein expression related to amino acid and fatty acid transport in the placenta
The mRNA expression of amino acid and fatty acid transporters in the placenta is shown in Fig. 6. Compared with the CON group, maternal dietary UR supplementation up-regulated mRNA expression of mTOR, PPARα and FABP3 of placenta (P < 0·05). However, maternal dietary UR supplementation decreased mRNA expression of transporters including excitatory amino acid transporters 2 (EAAT2), excitatory amino acid transporters 3 (EAAT3) and neutral AA transporter 1 (LAT1) in the placenta (P < 0·05). Moreover, mRNA expression of PPARγ in placenta was tended to decrease in the UR group (0·05 < P < 0·10).
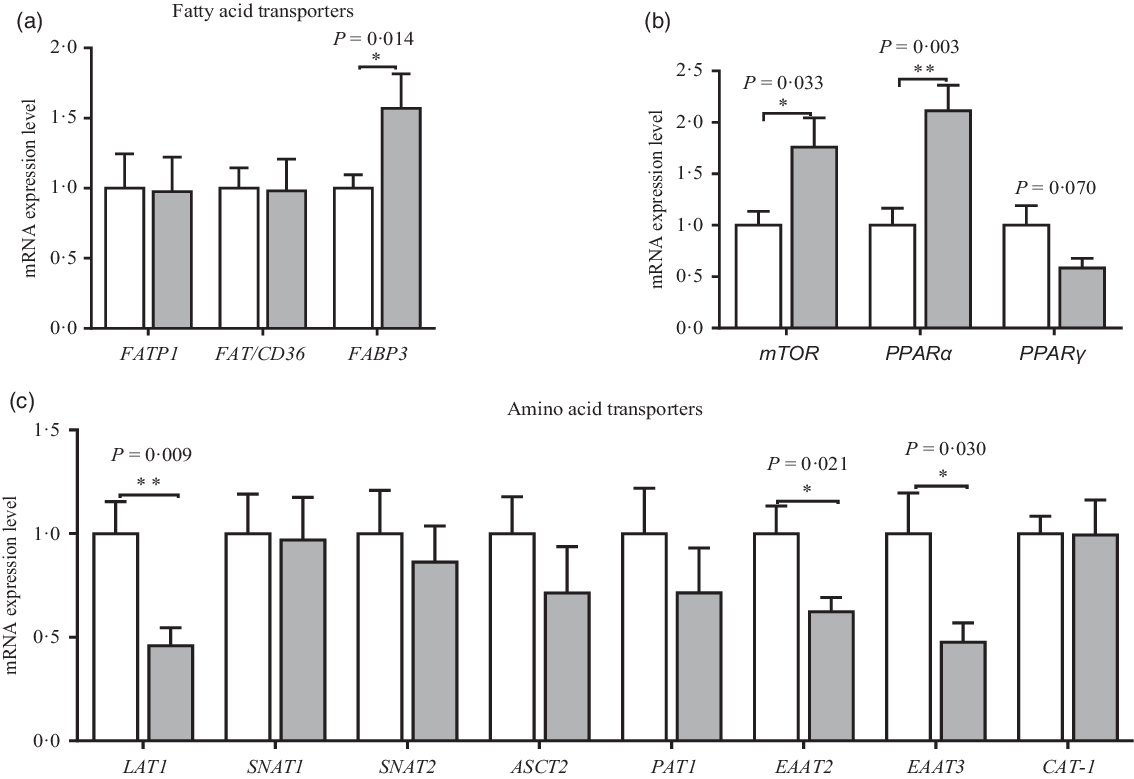
Fig. 6. Effects of maternal uridine (UR) supplementation at late pregnancy on mRNA expression of fatty acid (a) and amino acid (c) transporters in placenta. Data are mean values with their standard errors, n 8. Statistical significance was set at * P < 0·05 or ** P < 0·01 by t test. FATP1, fatty acid transport protein 1; FAT/CD36, fatty acid transporter/CD36; FABP3, fatty acid binding protein 3; mTOR, mammalian target of rapamycin; LAT1, neutral AA transporter 1; SNAT1, sodium-dependent neutral amino acid transporter 1; SNAT2, sodium-dependent neutral amino acid transporter 2; ASCT2, amino acid transporter 2; PAT1, proton coupled amino acid transporter 1; EAAT2, excitatory amino acid transporter 2; EAAT3, excitatory amino acid transporter 3; CAT-1, cationic amino acid transporter 1. ![]() , Control group;
, Control group; ![]() , UR group.
, UR group.
The relative protein expression of the mTORC1–PPAR signalling pathway in the placenta is shown in Fig. 7. Compared with the CON group, maternal dietary UR supplementation up-regulated protein expression of P-AKT, raptor, PPARα and PPARγ in the placenta (P < 0·05). In addition, the protein expression of phosphorylated-mTOR tended to increase in the UR group (P = 0·069).

Fig. 7. Western blot for the phosphorylated mammalian target of rapamycin complex 1 (mTORC1)–PPAR signalling pathway in placenta. Data are mean values with their standard errors, n 4. Statistical significance was set at * P < 0·05 or ** P < 0·01 by t test. AKT, protein kinase B; P-AKT, phosphorylated protein kinase B. ![]() , Control group;
, Control group; ![]() , uridine (UR) group. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
, uridine (UR) group. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
Maternal supplementation with uridine decreased birth mortality
Nucleotides in milk include adenosine 5′-monophosphate, cytidine 5′-monophosphate, guanosine 5′-monophosphate, inosine 5′-monophosphate and UMP, and UMP is the most abundant nucleotide in sows’ milk(Reference Stein, Peters and Mateo6). However, the de novo synthesis of UMP is always less efficient, a large part of UR is recycled and metabolised to UMP(Reference Ben-Sahra, Howell and Asara30). Our previous research showed that UR had higher bioavailability than UMP in early weaned piglets(Reference Li, Xie and Wang15). Maternal dietary UR supplementation reduced diarrhoea incidence in piglets by regulating the intestinal mucosal barrier and cytokine profiles(Reference Wu, Gao and Liu24), while this is the first research to study the effect of maternal dietary UR supplementation on reproductive performance in pigs. Previous studies reported that there is no effect on litter size and birth weight of piglets by supplementing yeast RNA with free nucleotides to sows 3 d before parturition(Reference Vitagliano, Bonato and Barbalho31). Besides, the addition of NuPro (a source of yeast-derived proteins) with no UMP to sows during late gestation is not sufficient to influence reproductive performance traits, including litter size, litter weight and mortality at birth(Reference Plante, Laforest and Farmer32). Thus, it was speculated that UMP or UR was the crucial nucleotides to fetal development, and the exogenous addition of UR to sows’ diet was beneficial to reduce mortality at birth.
Maternal supplementation with uridine-regulated amino acid content of sows and neonatal piglets
Amino acids were considered to be an important contributors for fetal growth and development(Reference Wu, Xie and Zhang33). The level of haematological indicators depends on the nutrition state, accumulated protein and fat reserves in the animal body(Reference Rekiel, Wie¸cek and Beyga34). In our study, dietary UR promoted protein synthesis of sows, caused by the increased serum total protein and albumin(Reference Rekiel, Wie¸cek and Beyga34). Besides, maternal dietary UR supplementation may regulate amino acid metabolism of sows and offspring because serum NH3 is mainly produced from amino acid metabolism, through the involvement of alanine aminotransferase and aspartate transaminase key enzymes in the process.
Maternal dietary UR supplementation affected amino acid contents and metabolism of sows and piglets through the following mechanisms. First, cysteine and methionine regulate oxidative status in the body, and methionine can be converted to cysteine(Reference Tian, Zeng and Zhang35). In this study, dietary UR supplementation improved the relative amount of cysteine and methionine in serum of sows, suggesting that UR could regulate the metabolism of sulphur-containing amino acids. Second, neutral amino acids, including tyrosine, phenylalanine, threonine and isoleucine, involve in the glycolipid and nucleotide metabolism of the body through mutual conversion or their metabolites. Conversely, UR may affect indirectly the metabolism of phenylalanine, tyrosine, threonine and isoleucine by regulating the expression of transporters or key enzymes. In addition, nucleotides can regulate the metabolism of arginine by enhancing the activity of glutamine synthetase(Reference Kosenko, Llansola and Montoliu36), and arginine can reduce fetal mortality in pigs(Reference Gao, Jiang and Lin37). In line with these reports, supplementing UR to sows improved the relative amount of arginine in the serum and decreased mortality at birth. The aforementioned results suggested that the increase of some amino acids might be owing to the AA-sparing effect by UR supplementation, and it is beneficial to protein synthesis of the sow, and this is consistent with the result of another study in grass carp(Reference Tie, Wu and Jiang9). Finally, in a mammal, pyrimidine nucleotide, including UTP, GTP and its metabolites, is degraded to β-alanine and β-aminobutyric acid(Reference Moffatt and Ashihara38). Our study observed that UR supplementation to sows improved the relative amount of β-alanine in the serum, suggesting that excess UR is excreted after being metabolised in the body.
Maternal dietary UR supplementation also regulated amino acid profile in the serum and liver of neonatal piglets. First, diet, the salvage pathway and direct de novo synthesis using amino acids are three ways to meet the body’s nucleotide needs in mammals(Reference Tie, Wu and Jiang9). In the process of de novo synthesis, glutamic acid, aspartic acid, alanine, glycine, serine and phosphoserine engaged in the synthesis of nucleotides and β-alanine and β-aminobutyric acid are involved in the metabolism of nucleotides(Reference Le, Urasaki and Pizzorno39). Thus, we speculated that optimal supplementation of UR to sows might inhibit fetal de novo synthesis of nucleotides and conserve some amino acids, which are consistent with previous reports(Reference Tie, Wu and Jiang9), thus, leading to these amino acids continued deposition in the liver of neonatal piglets by regulating related protein or transporters expression. On the other hand, excess UR is excreted after being metabolised in the neonatal piglets. Thus, the relative amount of glutamic acid, aspartic acid, phosphoserine, β-aminobutyric acid and β-alanine in the serum of neonatal piglets, and glutamic acid, aspartic acid, glycine, serine and alanine in the liver of neonatal piglets were improved. Second, the neonatal period is a rapid metabolic stage of energy and protein, and cysteine and histidine take part in the energy metabolism in the body; taurine and carnosine are their metabolites, respectively. As described above, maternal dietary UR could be involved in the metabolism of sulphur amino acids and promote energy metabolism and protein deposition in the liver of neonatal piglets by regulating the activity of the related enzyme. Thus, it is suggested that supplementation of UR to sows can regulate sulphur-containing amino acid metabolism, as well as some amino acids related to nucleotide metabolism of offspring.
To further unlock the effects of UR on amino acid metabolism, gene expression related to amino acid metabolism in the liver and placenta was detected. Na-dependent neutral amino acid transporter (SNAT) mediates the Na+-dependent uptake of small neutral amino acids including glutamine, histidine and alanine, methionine, threonine and key intermediary metabolites(Reference Gu, Roderick and Camacho40). Na-dependent neutral amino acid transporters (SNAT1 and SNAT2) are the most widely expressed genes in the liver. In this study, maternal UR supplementation up-regulated the mRNA expression of SNAT1 and SNAT2 in the liver of neonatal piglets, suggesting UR affected some amino acids transport in the liver by regulating the mRNA expression level of SNAT1 and SNAT2. Meanwhile, glutathione S-transferase A1 (GSTA1) is a key enzyme for glutathione synthesis, and it is involved in the metabolism of glutamic acid, cysteine and glycine. Thus, it was indicated that maternal UR supplementation may regulate the metabolism of some amino acids, such as cysteine, glycine, by up-regulating mRNA expression level of GSTA1. However, the causes still remained unknown and may require further investigation.
The placenta is the organ through which gases, nutrients and wastes are exchanged between the maternal–fetal circulations(Reference Belkacemi, Nelson and Desai41). Placental nutrient supply is adaptive to meet fetal growth demand, evidences suggested that adaptation of nutrients occurred in the regulation of transporter activity and expression(Reference Sibley, Brownbill and Dilworth42). During pregnancy, amino acids are involved in fetal development and growth(Reference Wu, Yin and Liu5). Thus, to further understand the mechanism of maternal dietary UR supplementation on placental amino acid transport, mRNA expression of placental transporters was investigated. The results revealed that maternal dietary UR supplementation down-regulated placental gene expression of transporters including PEPT1, EAAT2, EAAT3 and LAT1, implying that dietary UR to sows can inhibit the transfer of amino acid, which is consistent with our hypothesis that supplement UR to sows had amino acid sparing effect for sows, and fetal development is not affected in this condition.
Maternal supplementation with uridine-regulated fatty acid constituents of neonatal piglets
UR is a pyrimidine nucleoside, and it can regulate liver lipid metabolism and glycogen deposition(Reference Thuc, Yasuyo and Giuseppe43). During the last third of gestation, the maternal metabolic state is catabolism and maternal lipid and GLU breakdown quickly(Reference Jensen, Mølck and Lykkesfeldt44). Lipid is important to provide energy for the neonatal piglets; thus, UR plays a major role in glycolipid metabolism of sows and neonatal piglets. The study had demonstrated that UMP reduces plasma GLU level and increases TAG accumulation in the calves(Reference Katoh, Yoshioka and Hayashi45). In the present study, maternal dietary UR supplementation increased TAG, TBA and LACT, and decreased CHOL, HDL, LDL and GLU in the serum of sows, suggesting that dietary UR may have effect of suppressing serum GLU levels(Reference Yoshioka, Katoh and Hayashi46) by regulating glycolysis process of sows during parturition, and more likely caused by higher LACT in the serum of sows. In addition, dietary UR may regulate the formation of bile acids of sows during parturition, and more likely caused by higher TBA in the serum of sows, and TBA involves in the removal of UR from the body(Reference Deng, Wang and Gordillo11). However, maternal UR supplementation increased CHOL, HDL, LDL and LACT, and decreased TBA in the serum of neonatal piglets, implying that maternal dietary UR supplementation can regulate the metabolism of CHOL and TBA, which are required for the development of piglets and CHOL is particularly essential for embryogenesis(Reference Cai, Jia and Lu4).
Medium- and long-chain fatty acids play a key role in fetal development and growth. The previous study in newborn infants or rats showed that dietary nucleotides and their metabolites may enhance the synthesis of lipoproteins during the early neonatal period, influence the lipid metabolism and stimulate PUFA(Reference Sato, Murakami and Nakano47,Reference Sánchez-Pozo, Morillas and Moltó48) . In addition, another study in grass carp showed that dietary nucleotides up-regulated the level of C17 : 0, C20 : 0 and total SFA(Reference Tie, Wu and Jiang9), agreed with our study that maternal dietary UR supplementation increased fatty acids concentrations including C17 : 0, C20 : 0, C18 : 2N6C, total SFA and total PUFA in the liver of neonatal piglets. Nevertheless, maternal dietary UR supplementation decreased fatty acids concentrations including C18 : 1N9C, C20 : 1 and total MUFA in liver of neonatal piglets, which indicated that dietary UR to sows reduces the synthesis of MUFA of offspring. Thus, it was speculated that dietary UR to sows may promote transport, extension and desaturation of the fatty acid chain, as well as lipolysis by affecting the expression of key enzymes in the liver.
There are many key enzymes, such as ELOVL5, FADS1, CYP7a1 and HSL, which play a vital role in hepatic glycolipid metabolism. The results showed that maternal dietary UR supplementation up-regulated mRNA expression of FATP1, ELOVL5, FADS1, CYP7a1 and HSL, while down-regulated the mRNA expression of ACC and SREBP1c in the liver of neonatal piglets, which implies that dietary UR to sows affected the transportation and prolongation of the fatty acid chain, and mutual transformation between cholesterol and bile acid. Initially, UR promoted oxidative decomposition of fat and inhibited the synthesis of fatty acids by regulating mRNA expression of HSL and ACC because HSL and ACC are rate-limiting enzymes for lipolysis and synthesis, respectively. Second, UR may reduce the synthesis of CHOL and promote the conversion of CHOL in the liver by regulating mRNA expression of SREBP1c and CYP7a1 because SREBP1c and CYP7a1 are rate-limiting enzymes for the synthesis of cholesterol and bile acid, respectively(Reference Cai, Jia and Lu4). Finally, it was expected that UR promoted the transport of long-chain fatty acids and the prolongation of fatty acids in the liver by regulating mRNA expression of FATP1 and ELOVL5. Thus, maternal dietary UR supplementation could promote fat oxidation and inhibited the synthesis of fatty acids in the liver of neonatal piglets, which is essential for the energy supply of neonatal piglets. Medium- and long-chain fatty acids are efficiently absorbed and metabolised and help to improve the survival rate of neonatal piglets(Reference Enke, Seyfarth and Schleussner49).
Fatty acid translocase (FAT), fatty acid transport protein (FATP) and fatty acid binding protein (FABP) are involved in the transfer of placental fatty acids. In the present study, maternal dietary UR supplementation up-regulated placental mRNA expression of FABP3, suggesting that dietary UR to sows can promote the transfer of fatty acid, especially for PUFA thereby promoting the placental and fetal growth and development. In summary, maternal dietary UR supplementation promoted placental transfer of fatty acid and regulated fat metabolism of neonatal piglets by regulating gene expression of key enzymes or proteins.
Maternal supplementation with uridine-regulated protein expression level related to mTORC1–PPAR of placenta
The most vital function of placenta is the efficiency with which the placenta transfers nutrients. The mTOR complex 1 (mTORC1) is a key signalling pathway that regulates de novo synthesis of pyrimidine nucleotides and amino acid transport(Reference Robitaille, Christen and Shimobayashi50). In addition, as a nuclear receptor, PPAR regulates placental development and lipid metabolism(Reference Lager, Gaccioli and Ramirez51,Reference Martínez, Kurtz and Capobianco52) . Our previous study in early-weaned piglets showed that short-term UR treatment might regulate protein expression of mTOR and AKT pathways(Reference Zhang, Guo and Xie16). Similarly, we found that UR treatment could improve the phosphorylation of mTOR and AKT, raptor, which indicated that UR may regulate placental transfer through mTORC1 and AKT pathway. Interestingly, placental protein expression of PPARα and PPARγ was up-regulated by dietary UR supplementation to sows, signifying that dietary UR to sows regulates placental fat metabolism via up-regulating the expression of PPARα and PPARγ. Maternal supplementation with UR may affect the synthesis of pyrimidine nucleotides and thus influence the activity of mTORC1. Activated mTORC1 stimulates the activity of AKT, PPARγ and PPARα (Reference Cybulski, Polak and Auwerx53); thereby, the placental transfer function of amino acid and fatty acid was regulated. However, the effect of maternal UR supplementation on placental development needs further study.
Conclusion
In conclusion, maternal UR supplementation could regulate placental fatty acid and amino acid transport by regulating the mTORC1–PPAR pathway, influencing the fatty acid and amino acid constituents of neonatal piglets, thus reducing birth mortality and improving the reproductive performance of sows. These findings may be useful for greater production performance of sows and provide insight for mother–infant nutrition in humans.
Acknowledgements
We thank Dr Chun-yan Xie for participating in the experimental design and discussion.
The present study was supported by grants from the National Key R&D Program of China (2018YFD0501003), Jiangxi Provincial Innovation and Entrepreneurship Projects Science, Technology Projects of Hunan Province (2019RS3020 562 and 2019RS3021), and China Agriculture Research System (CARS-35).
The contributions of the authors were as follows: L. G., X. W. and Y. Y. designed the study. L. G., Y. L. and Y. Z. participated in the animal experiment. L. G. and Y. L. conducted the research and analysed the data. L. G., X. Z. and X. W. discussed the results and wrote the paper.
The authors declare that they have no conflicts of interest.