Anorectal malformations (ARM) refer to atresia or stenosis of the anus and rectum, with or without a fistula between the bladder, urethra, perineum or vestibule(Reference Godse, Best and Lawson1). The frequency of ARM appears to be roughly 4 per 10 000 births, though the frequency differs somewhat by country(2–Reference St Louis, Kim and Browne4). In 2013, the mortality from ARM in Japan was the lowest among the neonatal surgical diseases(Reference Yagi, Kohno and Asagiri5). Data from the North-East Italy Registry showed that the 10-year survival probability for isolated ARM was 100 %(Reference Cassina, Fascetti Leon and Ruol6). However, ARM require surgical repair, and infants with ARM may suffer from perioperative complications and typically repeated outpatient visits after discharge. Moreover, since parents know that their infants need surgical intervention in the first year of life, they often face lasting distress and anxiety and become exhausted with the long-term care of their infants(Reference Hinton, Locock and Long7). The patients themselves often suffer from active long-term problems, such as faecal incontinence, chronic constipation, urinary incontinence, ejaculatory dysfunction and/or erectile dysfunction(Reference Rigueros Springford, Connor and Jones8). Further, from the viewpoint of medical economics, it is impossible to overlook the financial burden of ARM, even if the condition is uncommon. In the USA, for example, the average total hospital charges for the primary repair of ARM in 2012 were estimated to be $72 631 per hospital stay(Reference Kovacic, Matta and Kovacic9). Thus, we should consider the possibility of primary prevention of ARM.
Insufficient maternal intake of folate is an established risk factor for neural tube defects(Reference Kondo, Matsuo and Morota10,Reference Tamura and Picciano11) . To prevent folate insufficiency among childbearing-aged women, staple foods have been fortified with folic acid in many countries and nationwide folic acid fortification resulted in a decline in the occurrence of neural tube defects(Reference Canfield, Collins and Botto12,Reference De Wals, Tairou and Van Allen13) . Interestingly, such fortification was likely to have also reduced the occurrence of non-neural tube defect anomalies(Reference Canfield, Collins and Botto12), and ARM may have been one of these latter types of anomaly. In a public health campaign conducted in China between 1993 and 1995, use of a 400-μg folic acid supplement was associated with a roughly 40 % decrease in the risk of ARM when compared with non-use(Reference Myers, Li and Correa-Villaseńor14). Also, an experimental study reported that Adriamycin-induced ARM occurrence decreased in rats supplemented with folic acid during pregnancy(Reference Faria, Simões Mde and Teixeira15). However, meta-analysis based on the results of seven epidemiological studies indicated that maternal use of folic acid supplement was not associated with ARM (OR 0·93, 95 % CI 0·77, 1·13)(Reference Zwink and Jenetzky16). In contrast, the use of vitamins or supplements that containing folic acid was associated with a reduced risk of ARM among women without diabetes mellitus, in a US multicentre case–control study(Reference Correa, Gilboa and Botto17), suggesting possible limitations in evaluating the independent effects of folate on ARM. Folate (vitamin B9) has a major role in the one-carbon metabolism related to the synthesis of purine and thymidine nucleotides, and the synthesis of methionine from homocysteine(Reference Li, Wahlqvist and Li18). Likewise, vitamins B6 and B12 act as enzyme cofactors for one-carbon metabolism(Reference Tamura and Picciano11,Reference Ebara19) ; for example, vitamin B6 is a cofactor in the conversion of tetrahydrofolate to 5,10-methylene tetrahydrofolate, and vitamin B12 works a cofactor in the conversion of homocysteine and 5-methyltetrahydrofolate to methionine and tetrahydrofolate, respectively. Thus, evaluation not merely of folate alone, but of the combination of vitamins B6, B9 and B12, appears likely to provide substantive new findings regarding the association between folate intake and ARM.
The purpose of this study was to explore the extent to which maternal intake of one-carbon metabolism-related B vitamins, including folate, vitamin B6 and vitamin B12, was associated with the risk of ARM. We hypothesised that a high combined intake of these three nutrients reduces the occurrence of ARM.
Methods
Study participants
The Japan Environment and Children’s Study (JECS), an ongoing nationwide birth cohort study, includes data on 103 099 pregnancies. From 2011 to 2014, women were recruited as early in pregnancy as possible (median = 12th week of gestation, interquartile range = 10, 15th) in fifteen Regional Centres throughout Japan. The concept and design have been previously described in detail(Reference Kawamoto, Nitta and Murata20). The respective distributions of maternal and infant characteristics in the JECS were comparable with those obtained in the national survey(Reference Michikawa, Nitta and Nakayama21). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving participants were approved by the Japan Ministry of the Environment’s Institutional Review Board on Epidemiological Studies (No. 100406001) and the Ethics Committees of all participating institutions. Written informed consent was obtained from all participants.
Of the 103 099 pregnancies, 95 170 unique mothers (excluding repeated registration) with subsequent delivery record were identified. In this study, we excluded participants who delivered infants with Down’s syndrome, trisomy 18 or trisomy 13, to exclude cases of ARM related to chromosomal factors(Reference Wang, Li and Cheng22) (further exclusions are summarised in online Supplementary Fig. S1), resulting in 89 235 mothers who delivered singleton live births being included in the analysis.
Dietary assessment
We distributed self-administered questionnaires twice, first during the first trimester (median fill-in week of gestation = 15), and then again during the second/third trimester (27th week of gestation). The first questionnaire included a FFQ regarding dietary intake in the preceding year, and the second a FFQ regarding the same items, but focusing on usual intake after awareness of pregnancy. In this study, we used data from the first FFQ as a marker of dietary intake in early pregnancy and treated it as a main exposure index reflecting exposure during the ARM developmental origin period. We used data from the second FFQ as a marker for mid-late pregnancy.
The FFQ used in the maternal surveys was developed for the Japan Public Health Centre-based prospective Study for the Next Generation and validated for 142 Japanese women aged 40–74 years, using a 12-d weighed food record(Reference Yokoyama, Takachi and Ishihara23). This FFQ focused on the frequency of consumption and portion size for each food item. The response choices for frequency were: <1 time/month, 1–3 times/month, 1–2 times/week, 3–4 times/week, 5–6 times/week, 1 time/d, 2–3 times/d, 4–6 times/d and ≥7 times/d; and those for portion size were: small (50 % less than standard), medium (equal to standard) and large (50 % more than standard). We calculated the daily food intake by multiplying the frequency by the standard-equivalent portion size, for each food item. The daily intake of nutrients, such as folate, vitamin B6 and vitamin B12, was estimated using the Standard Tables of Food Composition in Japan 2010(24). We adjusted for total energy intake using the residual model(Reference Willett, Howe and Kushi25).
The first and second questionnaires included a question about the frequency of taking folic acid supplement at the time, with seven response choices: none, once in a month, 2–3 times a month, 1–3 times a week, 4–6 times a week, once a day and twice or more times a day. We also collected information on the supplemental use of multi-vitamins (only information on use or non-use), including B vitamins, from preconception to 12 weeks of gestation, via face-to-face interviews. As there was a lack of information regarding amount of use, we did not include the nutrient intake from such supplements in the FFQ-based estimation. In Japan, there are no national food-fortification programmes involving folic acid.
Identification of anorectal malformations
Information on physician diagnoses of ARM was retrieved from the medical records. In accordance with the JECS in-house standard operating procedures, medical record transcriptions were performed three times by physicians, midwives/nurses and/or Research Coordinators: first during the first trimester, second after delivery and finally at the first-month health check-up after delivery. We used data from the forms after delivery and a month after delivery, which contained a list of sixty-one congenital anomalies, including ARM (10th edition of the International Classification of Diseases: Q42)(Reference Mezawa, Tomotaki and Yamamoto-Hanada26), and registered ARM occurrence when ARM were reported in either form. Information on the clinical classification of the respective ARM was not collected.
Statistical analysis
Based on a large sample size, an exploratory analysis on an observational study was done. Therefore, we did not perform a power calculation.
Given the small number of cases, we categorised the participants into two groups (high or low) based on the dietary assessment information. In the case of folate, women who consumed the estimated average requirement of dietary folate in Japan (≥400 μg/d)(27), or reported daily use of a folic acid supplement (once a day or more), were assigned to the high intake group. In Japan, daily folic acid supplements, with 400 μg of the monoglutamate form of folate, are typically available(28). In the case of dietary intake of vitamin B6 and B12, the median values were used for assignment to the high or low groups. Based on the B vitamin intake group data for early pregnancy, we summarised the following baseline characteristics of the 89 235 mothers: maternal age at delivery; smoking habits; alcohol consumption; pre-pregnancy BMI; current history of diabetes or gestational diabetes mellitus; infertility treatment; educational background; household income; occupation; use of multivitamin supplements; week of pregnancy at delivery; parity and infant sex.
The association of folate, vitamin B6 and B12 intake in early pregnancy with ARM occurrence was examined using logistic regression models, and the OR and 95 % CI of ARM, with the low intake group as the reference, were estimated. The first model was adjusted for maternal age at delivery. The multivariable model was further adjusted for the suspected risk factors of ARM, including smoking habits, alcohol consumption, pre-pregnancy BMI, diabetes or gestational diabetes mellitus and infertility treatment(Reference Boulet, Kirby and Reefhuis29,Reference Zwink, Jenetzky and Brenner30) . In the case of B6 and B12 intake, we additionally adjusted for use of folic acid supplement. To explore whether the combined intake of folate, vitamin B6 and vitamin B12 was associated with ARM occurrence, we defined three groups: low combined intake as reference (folate <400 μg/d, and low vitamin B6 and/or low vitamin B12), high intake (folate ≥400 μg/d, and high vitamin B6 and/or high vitamin B12) and remainder.
Through several sensitivity analyses, we confirmed whether consistent findings were obtained. First, we further adjusted for parity, hypertensive disorders of pregnancy, epilepsy during pregnancy(Reference Wijers, van Rooij and Bakker31), and protein, fish and vegetable intake in early pregnancy as possible confounders. Fish intake, which was inversely associated with congenital gastrointestinal tract atresia (oesophageal atresia, intestinal atresia and ARM) in our previous study(Reference Michikawa, Yamazaki and Ono32), was not included in the multivariable model because fish is a major food source of vitamin B6 and B12 in Japan(Reference Yoshino, Inagawa and Oshima33), and fish consumption in early pregnancy was correlated with the intake of these vitamins (Spearman’s correlation coefficient (ρ): 0·54 for vitamin B6 and 0·80 for vitamin B12). Vegetable intake was treated as a marker of healthy dietary habit. Second, we additionally adjusted for socio-economic status. Third, to avoid exposure misclassification, we excluded users of multi-vitamin supplements and participants with severe morning sickness. Fourth, the analysis was performed only on isolated cases of ARM (ARM with no other major congenital anomalies(34,Reference Parker, Mai and Canfield35) ), to exclude the potential influence of genetic factors. Fifth, further adjustment for combined intake of folate, vitamin B6 and vitamin B12 in mid-late pregnancy was conducted, to focus on the independent association between intake in early pregnancy and ARM.
Additionally, we repeated the above analyses, to examine the association of folate, vitamin B6 and vitamin B12 intake in mid-late pregnancy with ARM occurrence. For this intake period, we excluded mothers who did not have data from the second FFQ and delivered their infants at 22–27 weeks of gestation, leaving a total of 88 234 mothers available for analysis. The present study used the data set jecs-ag-20160424, which was released in June 2016 and revised in October 2016, along with the supplementary data set jecs-ag-20160424-sp1. All analyses were performed with Stata 15 (StataCorp LP).
Results
Among the 89 235 participants (mean age at delivery = 31·2 years), forty-three women delivered infants with ARM (4·8 per 10 000 live births). Overall, the median dietary intakes in early pregnancy were 245·4 μg/d for folate (estimated average requirement in Japan: 400 μg/d), 1·0 mg/d for vitamin B6 (1·2 mg/d) and 3·8 μg/d for vitamin B12 (2·3 μg/d); 28·3 % of the participants used a daily folic acid supplement in early pregnancy.
Table 1 shows the distribution of mothers’ baseline characteristics for the folate, vitamin B6 and vitamin B12 intake groups. In the case of folate, the high dietary and supplemental intake group showed a higher percentage of women with the following characteristics, compared with the low intake group: age at delivery ≥35 years, never-smoked, infertility treatment, educational background ≥13 years, household income ≥6 million Japanese-yen/year, use of multi-vitamin supplements and nulliparae. In online Supplementary Table S1, we summarise the baseline characteristics of the mothers who delivered infants with ARM.
Table 1. Baseline characteristics of 89 235 pregnant women, in terms of maternal intake of folate, vitamin B6 and vitamin B12 in early pregnancy, Japan Environment and Children’s Study (2011–2014)
(Numbers and percentages; medians)
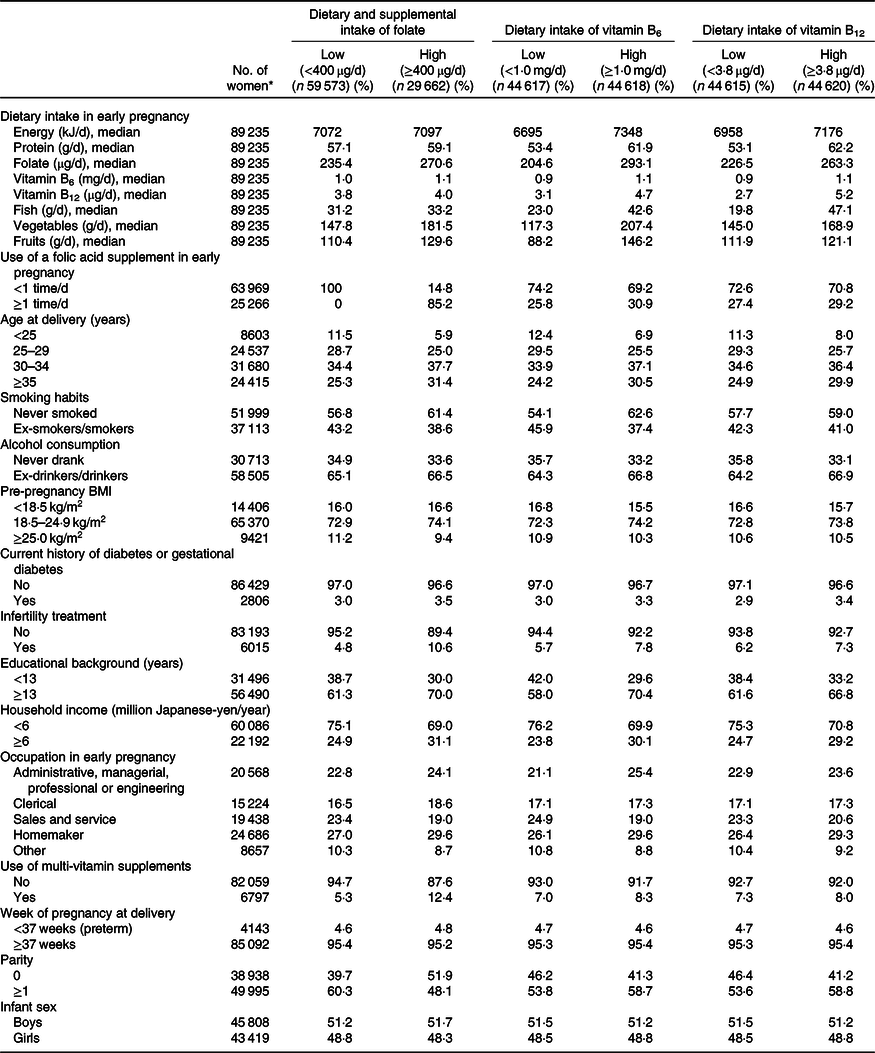
* Subgroup totals do not equal the overall number because of missing data.
Table 2 shows the association between the respective early pregnancy intakes of B vitamins and ARM. High vitamin B6 intake was non-significantly associated with decreased odds of ARM occurrence (adjusted OR for high v. low group = 0·5, 95 % CI 0·3, 1·0). Likewise, the estimated OR for folate and vitamin B12 intake showed a direction of lower risk. In terms of the association between combined B vitamin intake and ARM, the adjusted OR in the high combined intake group was 0·4 (95 % CI 0·2, 1·0) compared with the low intake group (Table 3); and the estimated OR in this high group did not vary substantially in the various sensitivity analyses.
Table 2. Anorectal malformation, in terms of maternal intake of folate, vitamin B6 and vitamin B12 in early pregnancy, Japan Environment and Children’s Study (2011–2014)
(Odds ratios and 95 % confidence intervals)

* OR were estimated using logistic regression model that included maternal age at delivery, smoking habits, alcohol consumption, pre-pregnancy BMI, current history of diabetes or gestational diabetes and infertility treatment. Participants with missing values for these factors were excluded, which left 89 034 in the multivariable models.
† Additionally adjusted for use of folic acid supplement in early pregnancy.
Table 3. Association between combined intake of folate, vitamin B6 and vitamin B12 in early pregnancy and anorectal malformation (ARM)
(Odds ratios and 95 % confidence intervals)
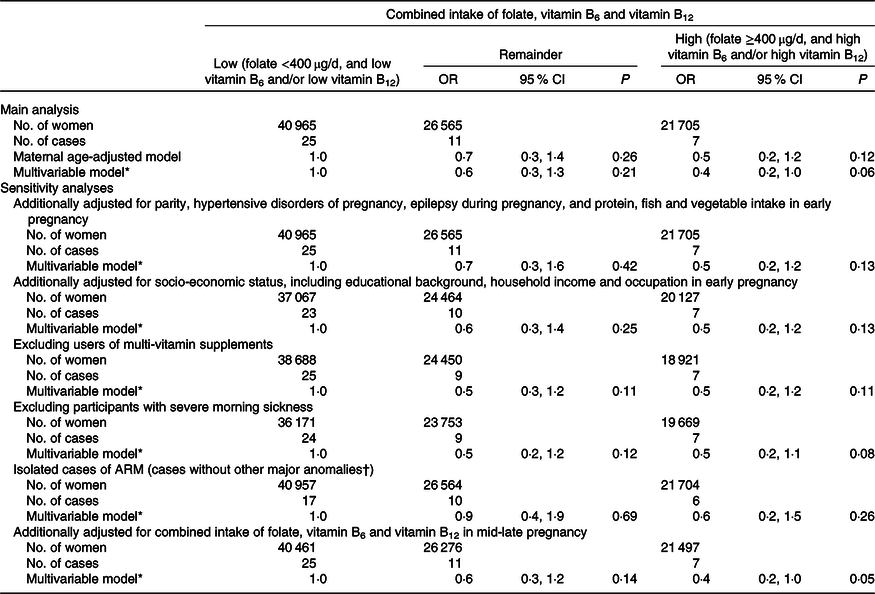
* OR were estimated using logistic regression model that included maternal age at delivery, smoking habits, alcohol consumption, pre-pregnancy BMI, current history of diabetes or gestational diabetes and infertility treatment.
† Major anomalies included anencephaly, spina bifida, encephalocele, microphthalmia, cleft palate, cleft lip (with or without cleft palate), congenital heart disease (not including patent ductus arteriosus), gastroschisis, omphalocele, diaphragmatic hernia, oesophageal atresia, small intestinal atresia, hypospadias and reduction defects of the upper and/or lower limbs(34,Reference Parker, Mai and Canfield35) .
Table 4 shows the association between the respective mid-late pregnancy intakes of B vitamins and ARM. B vitamin intake in this period was moderately correlated with that in early pregnancy (ρ = 0·57 for folate, 0·58 for vitamin B6 and 0·48 for vitamin B12) (online Supplementary Table S2). However, there was no association observed between ARM and B vitamin intake, either individually or in combination, in this period.
Table 4. Anorectal malformation, in terms of maternal intake of folate, vitamin B6 and vitamin B12 in mid-late pregnancy, Japan Environment and Children’s Study (2011–2014)*
(Odds ratios and 95 % confidence intervals)
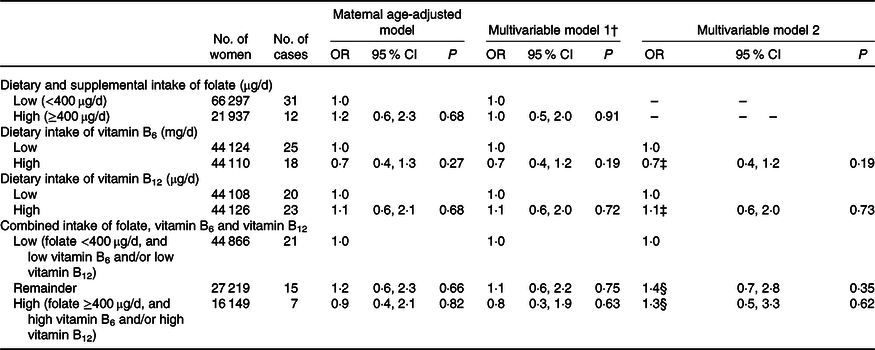
* We included 88 234 women who had valid data on a FFQ during the second/third trimester and delivered their infants after 28 weeks of gestation. OR were estimated using logistic regression model.
† Adjusted for maternal age at delivery, smoking habits, alcohol consumption, pre-pregnancy BMI, current history of diabetes or gestational diabetes and infertility treatment. Participants with missing values for these factors were excluded, which left 88 049 in the multivariable model.
‡ Additionally adjusted for use of folic acid supplement in mid-late pregnancy.
§ Additionally adjusted for combined intake of folate, vitamin B6 and vitamin B12 in early pregnancy.
Discussion
In this prospective analysis of mothers recruited throughout Japan, the combined intake of folate, vitamin B6 and vitamin B12 (rather than the individual intake of any one of these) appeared to be associated with reduced risk of their infants having ARM. This association, however, was only observed in early pregnancy, not in mid-late pregnancy. Given that the embryonic period (the first 8 weeks) is important in terms of the developmental origins of ARM(Reference Gupta, Bischoff and Penñ36), the observed tendency towards an inverse association with combined B vitamin intake in early pregnancy did not conflict with our hypothesis.
The JECS includes one of the largest birth cohorts in the world(Reference Michikawa, Nitta and Nakayama21). The frequency of ARM in the JECS population (4·8 per 10 000 live births) was comparable to past reports(2–Reference St Louis, Kim and Browne4); however, the number of outcome cases (forty-three) was small from the viewpoint of statistical rigour, and the possibility of chance findings remains. However, we should not underestimate the strength of the association; the estimated OR was 0·4 among mothers in the high combined intake group, compared with those in the low intake group. In this case, the E-value, which indicates the robustness of a given association with respect to potential unmeasured confounders(Reference VanderWeele and Ding37), was 4·4. Thus, a confounder that was associated with B vitamin intake or ARM occurrence with a more than 4·4-fold relative risk might explain away the association here observed between B vitamins and ARM. One of the unmeasured possible confounders is infection-related fever during pregnancy; however, a large European case–control study reported that the adjusted OR for fever during the first 4 months of pregnancy was 2·2 (95 % CI 0·8, 5·7)(Reference Wijers, van Rooij and Bakker31). Although, given the nature of observational investigation, the present study also had unmeasured confounders, the existence of such a significant confounder seems unlikely. With respect to measurement error which the E-value cannot evaluate, such error would naturally occur in dietary assessment based on self-reporting, and in the use of a FFQ not validated specifically for pregnant women, both of which apply to this study. However, such error would likely have here led to non-differential misclassification that would have weighted the OR point estimates towards null values, whereas an inverse association was suggested. Further, the estimated B vitamin intakes for the studied population did not deviate from the overall national estimate for women (median = 253 μg/d for folate, 0·97 mg/d for vitamin B6 and 3·7 μg/d for vitamin B12 in the National Health and Nutrition Survey 2013(38)). Based on careful interpretation of the results, we concluded that there was a meaningful association between combined B vitamin intake and the occurrence of ARM.
A study in China between 1993 and 1995 reported a marginally decreased risk of ARM with regular folic acid supplementation (400 μg/d)(Reference Myers, Li and Correa-Villaseńor14), and a population-based case–control study in Hungary showed evidence of a preventive effect against ARM, through maternal folic acid supplementation(Reference Czeizel, Tóth and Rockenbauer39). However, no association between the use of folic acid and ARM was found in a number of observational studies(Reference Zwink, Rissmann and Potzsch40–Reference Källén43), and dietary and supplemental folate intake was not associated with ARM occurrence in the present study. We had a related interest in the difference in ARM occurrence with the intake of multi-vitamins containing and not containing folic acid. A Netherlands case–control study based on 371 cases found no association with folic acid use (only 22 % of users also took multi-vitamins)(Reference Wijers, de Blaauw and Zwink41), whereas a US case–control study based on 511 cases reported that intake of vitamins or supplements with folic acid was associated with reduced ARM risk among non-diabetic women(Reference Correa, Gilboa and Botto17). This suggests the possibility there is a combined effect of folate and its related nutrients on ARM pathogenesis, rather than folate alone. We thus hypothesised that the disturbance of folate (or ‘one-carbon’) metabolism, which has been suggested as the pathogenesis of neural tube defects(Reference Li, Wahlqvist and Li18), had a role in ARM occurrence. Supporting the hypothesis of the present study, the study results suggested that combined intake of folate, vitamin B6 and vitamin B12 tended to be protectively associated with ARM occurrence. There is evidence that supplements combining folic acid and vitamins B6 and B12 reduced the homocysteine plasma level(Reference Lonn, Yusuf and Arnold44). Given a low intake of B vitamins, impairment of one-carbon metabolism leads to homocysteine accumulation, which increases oxidative stress(Reference Li, Wahlqvist and Li18,Reference Tyagi, Sedoris and Steed45) and impairs nucleotide biosynthesis. These may inhibit the development of hindgut (the origin of the lower gastrointestinal tract). Certainly, oxidative stress seems to be involved in the pathogenesis of obesity- and diabetes-related congenital anomalies, including ARM(Reference Zwink, Jenetzky and Brenner30,Reference Carmichael, Rasmussen and Shaw46) . However, no interaction was observed between folic acid use and methylenetetrahydrofolate reductase C677T polymorphism, in association with ARM(Reference Wijers, de Blaauw and Zwink41). The one-carbon metabolism pathway is a candidate for the underlying ARM mechanism, but would only partially account for the ARM pathogenesis. Since the pathogenesis of ARM is unclear, further studies, which focus on one-carbon metabolism, may contribute to its elucidation.
To the authors’ knowledge, no birth cohort study has reported on differences in ARM occurrence between mothers who had high or low combined B vitamin intake. In addition, we used a nationwide cohort assumed to be representative of Japanese pregnant women(Reference Michikawa, Nitta and Nakayama21), whose frequency of pre-conceptional folic acid use is typically lower than that of women in western countries(Reference Ishikawa, Obara and Nishigori47). Further, since we prospectively collected information on nutritional intake during pregnancy, we could ignore differences in the degree of recall bias between mothers who delivered infants with and without ARM, and the temporal association between exposure and outcome was warranted. Despite these strengths, we again note the limitation represented by the small number of ARM cases, which resulted in a broad 95 % CI for the OR point estimates, and should acknowledge some additional limitations of this study. One lay in the supposition that the women’s usual dietary habits continued in early pregnancy. Since the embryonic period overlaps with the period of morning sickness, one’s dietary habits might change in early pregnancy. Thus, we performed a sensitivity analysis that excluded participants with severe morning sickness and confirmed that this factor did not substantially affect the result. Another limitation is that we did not assess dietary methionine intake (a regulator of one-carbon metabolism). If methionine intake had been included in the analysis, it might have enabled more in-depth discussion of the contribution of one-carbon metabolism to ARM pathogenesis. Thirdly, we did not have information on ARM diagnosed after the first month after birth; however, most ARM cases are diagnosed during the newborn period(Reference Levitt and Pena48). Also, we did not collect information on the clinical classification of ARM. Finally, our analysis was restricted to women who delivered live births. This restriction, however, was unlikely to affect the target association because the majority of stillbirths with ARM appears to be caused by genetic and/or chromosomal factors.
In conclusion, we observed a suggestive inverse association between intake of one-carbon metabolism-related B vitamins in early pregnancy and ARM occurrence. Future research, focused on one-carbon metabolism, seems likely to contribute to elucidation of the pathogenesis of ARM.
Acknowledgements
We would like to express our gratitude to all of the JECS study participants and participating Co-operating health care providers.
The Japan Environment and Children’s Study was funded by the Ministry of the Environment, Japan. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Ministry of the Environment, Japan.
T. M., H. N. and S. Y. designed this study; T. M., H. N., M. S. and S. Y. contributed to the data analysis; T. M., H. N., S. F. N., T. I., E. S. and T. Kawamoto contributed to the data collection; T. M. wrote the initial draft of the manuscript; T. Kuroda and T. Kawamoto provided study supervision; All authors contributed to the interpretation of data, provided critical revisions of the manuscript and approved submission of the final manuscript.
None of the authors has any conflicts of interest to declare.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520001816
Appendix
Members of the JECS Group as of 2019: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Hiroyasu Iso (Osaka University, Suita, Japan), Masayuki Shima (Hyogo College of Medicine, Nishinomiya, Japan), Youichi Kurozawa (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan) and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).









