Maternal and neonatal health issues are in the spotlight again in the Sustainable Development Goal global agenda(1). Given that the target is to reduce mortality in the mother and the newborn, it is important to understand which maternal and newborn characteristics constitute risks at the time of birth. In 2010, one million infant deaths occurred at the time of birth and around 2 million deaths occurred during the neonatal period(Reference Liu, Johnson and Cousens2,3) .
Several health indicators can be used to analyse the quality of care and to evaluate the characteristics that may identify potential threats to the mother and newborn. One of the most commonly used indicators is severe morbidity or near miss. In 2011, the WHO published a guideline for the near-miss approach to improve maternal health. A maternal near miss was defined as a woman who nearly died but survived a complication that occurred during pregnancy, childbirth or within 42 d of termination of pregnancy(4). Based on the same concept, the neonatal near miss has been defined in the literature as an infant who nearly died but survived a severe complication that occurred during pregnancy, birth or within 7 d of extra-uterine life(Reference Pileggi, Souza and Cecatti5–Reference Pileggi-Castro, Camelo and Perdoná7).
Some maternal characteristics also have the potential to signal risk at the time of birth. The BMI is frequently calculated in prenatal consultations (body weight (kg)/height (m2)) to assess risk of adverse perinatal outcomes not only for the mother but also for the baby. Its importance is based on the fact that the maternal nutritional status is directly related to energy and micronutrient reserves and might interfere in fetal development and neonatal nutritional status(Reference De, Saunders and Leal8). Maternal weight and height data allow an easy and effective follow-up of the pregnant woman’s nutritional status.
Abnormal nutritional status is a concern in maternal health as there are risks related to lack of or excess weight gain during the gestational period. The BMI alone can be one indicator of the nutritional status in all stages of pregnancy including the moment of birth. It is not an accurate measure when used in a single moment but, in some settings, this is the only available data. Currently, there is only one chart to classify the women’s weight along pregnancy and this can be used as a nutritional status in a transversal analysis(Reference Atalah, Castillo and Castro9).
The WHO ‘Better Outcomes in Labour Difficulty’ (BOLD) project was a prospective cohort study of labour events and outcomes in 10 000 African women from Uganda and Nigeria that was designed with the aim of developing labour monitoring tool to accelerate the reduction of maternal and perinatal mortality(Reference Souza, Oladapo and Bohren10). This secondary analysis aimed to understand how the population behaved in relation to weight and to explore the correlation between maternal BMI and severe maternal and neonatal outcomes. We hypothesised that malnutrition (undernutrition, overweight and obesity) can negatively influence outcomes in women and their babies, especially in near-miss cases.
The main objective of this secondary analysis was to describe the nutritional status of the BOLD project study population and determine possible associations between maternal nutritional status (as reflected by maternal BMI at the time of birth) and severe neonatal outcomes (SNO). We also analysed previous maternal pathology and that in the index pregnancy to determine associations with neonatal outcomes.
Methods
Data collection took place in thirteen health facilities in Africa (nine in Nigeria and four in Uganda) over a period of 12 months (December 2014–November 2015). The facilities were chosen based on the number of births and on the care provided (e.g. professional capacity, having access to a Caesarean section, good labour practices, among others). Inclusion criteria for the women were singleton pregnancy, admission at first stage of spontaneous labour, cervical dilatation of 6 cm or less and informed consent. Exclusion criteria were fetal death, advanced first stage of labour, multiple pregnancy, gestational age less than 34 weeks, elective Caesarean section, Caesarean section before labour, minors without a guardian or who were not emancipated and those incapable of giving consent. The data were managed with RedCap(Reference Harris, Taylor and Thielke11). This is a secondary analysis of the BOLD project database, and details about the population and data collection instrument were published elsewhere(Reference Souza, Oladapo and Bohren10).
For this secondary analysis, we applied the criteria presented in Box 1 to identify maternal and neonatal near-miss cases in the study population. If a woman or a baby had one or more of the presented characteristics, the woman or baby was considered a case of near miss.
Box 1. Criteria used to classify maternal and neonatal near miss

We used the classification designed by Atalah et al. to determine the nutritional status of pregnant women in the cohort based on labour admission data. To the best of our knowledge that is the only such classification designed by Atalah et al. (Reference Atalah, Castillo and Castro9) (Table 1). We also used the Hyperglycaemia and Adverse Pregnancy Outcome study classification of maternal BMI at 28 weeks in order to compare data with this study(12). Women’s weight and height measurements were taken at the time of hospitalisation (when women entered the facilities in the study). The weight gained during the index pregnancy was not assessed. To describe the nutritional status of this population, figures of distribution and test of normality related to weight and BMI were presented for both women and babies.
Table 1. Maternal BMI (kg/m2) classification in the studied population*

* We used a modified classification designed by Atalah et al. (Reference Atalah, Castillo and Castro9).
SNO was defined in this study as neonatal near miss or death. This was done to create a dichotomous value to perform the test, once the original near-miss criteria were applied to predict death and to increase the number of severe outcomes in newborns. To explore the association between maternal BMI data and SNO, the χ 2 test was performed (P < 0·05). We also calculated the OR, 95 % CI and P value.
To identify a maternal characteristic or a group of characteristics that could predict SNO, we used Fisher’s exact test, the OR and the 95 % CI using previous maternal pathology collected in the BOLD project as well as that in the index pregnancy.
Results
We analysed 10 203 participants who were eligible for this study. The flowchart for the data sample is shown in Fig. 1. Descriptive analysis is shown in Table 2.

Fig. 1. Flowchart for data sample. BOLD, Better Outcomes in Labour Difficulty.
Table 2. Descriptive analysis of women and newborns in the present study
(Numbers and percentages)

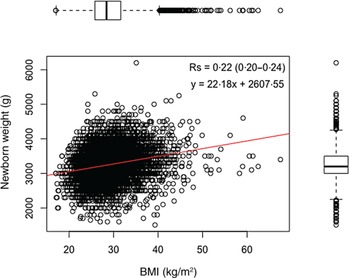
Fig. 2 shows the frequency distribution of newborn birth weight, and Fig. 3 shows the frequency distribution of maternal BMI. Both figures were merged in Fig. 4 and showed a weak correlation between data (Spearman’s correlation coefficient = 0·22, 95 % CI 0·20, 0·24).

Fig. 2. Birth weight distribution of newborns in the study population.

Fig. 3. Women’s BMI in the study population.

Fig. 4. Dispersion of maternal BMI by the weight of newborns in the study population.
Using a χ 2 test, there was no association between maternal BMI category at the time of birth and SNO both using Atalah and the Hyperglycaemia and Adverse Pregnancy Outcome study (P > 0·05).
Table 3 shows previous maternal pathology and Table 4 shows pathology in the index pregnancy in association with SNO using Fisher’s exact test. Table 3 shows that women with previous chronic hypertension, diabetes and obesity had a higher risk of having a baby with near miss or death. Table 4 shows that women in the index pregnancy with other obstetric haemorrhage, pre-eclampsia (without eclampsia), anaemia or gestational diabetes had a higher risk of having a baby with near miss or death.
Table 3. Analysis of association between maternal pathologies previous to the pregnancy with severe neonatal outcomes (near miss + death)
(Odds ratios and 95 % confidence intervals)

Table 4. Analysis of association between gestational pathologies with severe neonatal outcomes (near miss + death as a unique category)
(Odds ratios and 95 % confidence intervals)

Discussion
This study found that BMI at the time of birth was not associated with neonatal near miss or death, but women who were obese prior to pregnancy were more likely to experience adverse neonatal outcomes.
The BMI classification using Atalah’s curves in the moment of birth could be one possible explanation for the lack of association between the maternal BMI and severe outcomes. The curves of Atalah et al. (Reference Atalah, Castillo and Castro9) studied Chilean women as reference; they are not international or population-based curves; therefore, the risk of not being adequate for the present study population was high. This bias, although considered, cannot be attenuated since there are no other available curves. Cheikh et al. (Reference Cheikh Ismail, Bishop and Pang13) are conducting a multicentre project to develop a new curve to classify pregnant women according to gestational age. It is expected that the analyses will be redone with the availability of this new curve.
The Hyperglycaemia and Adverse Pregnancy Outcome study used a classification to BMI at 28 weeks of gestational age that follows: underweight <22·6, normal 22·6–28·4, overweight 28·5–32·9 and obese >33 kg/m2. With this classification, they found that greater BMI was associated with pregnancy complications such as primary Caesarean section, birth weight >90th percentile and neonatal hyperinsulinaemia(12). We used the same classification in order to compare our results with this large cohort, but we were not able to find an association with neonatal adverse outcomes.
One North American study including women with BMI greater than 60 kg/m2 at the time of delivery showed an increased risk for neonatal morbidity and maternal complications when compared with other obese women (BMI 30 kg/m2 or greater). In this study, they did not use any pregnancy BMI classification and they did not compare with eutrophic women at the time of delivery. They also had smaller odds of neonatal morbidity with less obese women and this could corroborate with our study once our sample size did not have many obese women(Reference Kim, Burn and Bangdiwala14). Despite this, other studies showed that overweight and obese mothers are more likely to have maternal and neonatal complications.
A 2016 review found that being overweight or obese pre-pregnancy increased the risk of admission to neonatal intensive care unit and birth of low birth weight babies, among others. These suggested that women should maintain normal BMI before pregnancy(Reference Liu, Xu and Wang15). A data meta-analysis of thirty-nine cohorts (Europe, North America and Oceania) found that higher pre-pregnancy BMI and gestational weight gain were associated with greater risks of maternal complications during pregnancy and preterm birth(Reference Santos, Voerman and Amiano16). In our study, we found similar results once previous obesity was associated with severe outcomes.
We found that previous histories of maternal obesity, diabetes and chronic hypertension were associated with SNO (near miss or death). According to Shaw et al., one in five pregnant women is obese or overweight and this may increase the risk of congenital anomalies, venous thromboembolism, pre-eclampsia, gestational diabetes, postpartum haemorrhage and Caesarean section(Reference Shaw, Guise and Shah17). Maternal obesity was also associated with sepsis and neonatal morbidity(Reference Rastogi, Rojas and Rastogi18).
The distributions of maternal BMI at the time of birth and the weights of newborns, as well as the dispersion of these two variables, were determined. Understanding how the distribution in a population behaves is important to the development of policies to improve health conditions. In the case of birth weight, an English study showed that the contemporary trend was for increasingly heavy babies, one reason being the increase in maternal age as well as an increase in incidence of obesity(Reference Ghosh, Berild and Sterrantino19). This was also seen in this study population, with nearly half being either overweight or obese.
A French study also observed this weight-related increase in its population(Reference Diouf, Charles and Blondel20). The authors found a 9·2 % prevalence of babies that were small for gestational age, with 11·5 % for those large for gestational age. In addition, they analysed maternal weight at the time of delivery (67·5 kg) and the height (1·617 m) that yielded a BMI equal to 25·81 kg/m2. We could not determine whether the results were similar, but the present study showed a higher mean BMI (29·11 kg/m2), which could represent greater risk for both the mother and baby.
The weight gain during pregnancy was one of the variables not reported in the BOLD project because it was not a study objective. Nevertheless, its importance cannot be ignored. The National Academy of Medicine recommends a range of weight gain that should be maintained throughout the gestational period(21). As it is likely that Nigeria and Uganda are undergoing a maternal and neonatal nutritional transition for weight gain, it is imperative that attention be given not only to intrapartum but also to prenatal care.
Women with previous obesity, diabetes and chronic hypertension, as well as those with gestational diabetes, anaemia, pre-eclampsia or other obstetric haemorrhage in the current pregnancy had significantly higher risk of SNO. This is well described in the literature(Reference Bramham, Parnell and Nelson-Piercy22–Reference Xiong, Saunders and Wang27).
This study had other limitations. We could not access maternal weight in a previous pregnancy or the weight gain during the index pregnancy for this analysis. This information could have improved the analysis and helped us understand the population better.
In addition, there were no pregnant women who gave birth at gestational age less than 34 weeks in this study, which reduced the scope of the neonatal near-miss concept. It is important to say that all women recruited for this study were low-risk pregnant women. However, the combined set had the necessary accuracy for the neonatal near-miss classification; this fact implies that the lower conceptual coverage is not necessarily negative.
Conclusion
This secondary analysis of the BOLD project found no association between maternal BMI at the time of birth and SNO. Despite this, to the best of our knowledge, it is important to notice that there is no accurate curve for pregnant women at the time of birth. With the present data, we could associate women with previous obesity, diabetes and chronic hypertension as well as those with gestational diabetes, anaemia, pre-eclampsia or other obstetric haemorrhage in the current pregnancy had significantly higher risk of SNO. These findings are already well described in the literature and we could corroborate them here.
Ethical statement
Scientific approval for the original study was obtained from the Review Panel on Research Projects (RP2) of the Department of Reproductive Health and Research, WHO. Ethics approval was obtained from the WHO Ethics Review Committee (protocol A65879), the Makerere University School of Health Sciences Research and Ethics Committee, Uganda (protocol no. SHSREC reference 2014-058), University of Ibadan/University College Hospital Ethics Committee (UI/EC/14/0223), Federal Capital Territory Health Research Ethics Committee, Nigeria (protocol FHREC/2014/01/42/ 27-08-14) and Ondo State Government Ministry of Health Research Ethics Review Committee, Nigeria (AD 4693/160).
Data can be made available upon request to the Department of Reproductive Health and Research, WHO, Geneva, Switzerland.
Acknowledgements
The BOLD project implementation was a collaborative effort of a large number of academic staff members, hospital personnel and researchers from thirteen hospitals in Uganda and Nigeria and a technical advisory group of international experts convened by the WHO. We acknowledge the contributions of all midwives, doctors, facility administrators and maternity unit staff of all hospitals that participated in the project. We would also like to acknowledge the assistance of Bukola Fawole and Kidza Mugerwa with the reviewing and editing of the article. For Professor Bukola Fawole, in memoriam. With his example and kindness, he inspired us. Bukola will never be forgotten. The manuscript presents the views of the named authors only.
This secondary analysis was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The original work was funded by the Bill & Melinda Gates Foundation (grant no. OPP1084318), The United States Agency for International Development (USAID) and the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a co-sponsored programme executed by the WHO. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors’ contributions were as follows: V. N. P., J. S. C., C. P. C. and J. P. S. conceptualised the study; V. N. P., J. S. C. and H. C. C. S. performed the formal analysis; V. N. P. and J. S. C. wrote the original draft; V. N. P., J. S. C., O. T. O., J. P. S., A. L. A., C. P. C., H. A. I., L. O. O., A. O. A. and H. C. C. S. reviewed and edited the manuscript.
All authors have no competing interests.