Chronic exposure of rodents to hyperenergetic diets can impair learning and memory( Reference Stranahan and Mattson 1 , Reference Winocur and Greenwood 2 ). Such diet-induced memory impairments have largely been shown for hippocampal-dependent spatial tasks and less so for perirhinal-dependent object discrimination( Reference Heyward, Walton and Carle 3 – Reference Davidson, Hargrave and Swithers 10 ). There is some evidence that obesity induced by chronic sucrose or high fat feeding to rats impairs performance in object recognition memory tests that measure the extent to which animals can discriminate between novel and familiar objects( Reference Jurdak and Kanarek 11 , Reference Ennaceur and Delacour 12 ). Therefore, it is well established that obesogenic diets influence a range of behaviours in rats. There is now great interest in knowing whether exposure to similar diets during early life can have similar effects. A number of studies have focused upon exposures during fetal life or early-neonatal stages. In rats, maternal obesity, due to overfeeding, can impair reversal learning( Reference Wu, Deng and Li 13 ). Interestingly, and in contrast to the detrimental effects of adult high-fat diet feeding( Reference Winocur and Greenwood 2 ), maternal obesity had a positive impact on spatial water-maze learning in the offspring when tested in adulthood( Reference Bilbo and Tsang 14 ). In contrast, maternal obesity due to high fat feeding seems to interfere with operant learning in adulthood( Reference Rodriguez, Rodriguez-Gonzalez and Reyes-Castro 15 ) and spatial learning is also impaired in the offspring from obese mice( Reference Tozuka, Kumon and Wada 16 ).
While the effects of early-life exposure to high-fat or high-sugar diets have been documented, less is known about the behavioural effects of Western-type diets such as the cafeteria diet (CD)( Reference Sclafani and Springer 17 – Reference Prats, Monfar and Castella 20 ). The CD, when compared with a high-fat diet, is particularly effective in modelling obesity-related metabolic abnormalities( Reference Sampey, Vanhoose and Winfield 21 ). A direct comparison of the CD and a high-fat diet also revealed differences in their effects on memory, suggesting differences between these diets beyond the induction of obesity( Reference White, Pistell and Purpera 22 ). Early developmental stages are a sensitive period for inducing long-lasting effects of cafeteria feeding on metabolism( Reference Bayol, Simbi and Stickland 23 – Reference Akyol, McMullen and Langley-Evans 25 ). However, little is currently known about the behavioural effects of early cafeteria feeding. A study by White et al. ( Reference White, Pistell and Purpera 22 ) has demonstrated that exposure to the CD or a high-fat diet had different sensitising effects on water-maze retention following a re-exposure to the same diet in adulthood. We have recently demonstrated that early, in particular lactational, cafeteria feeding does not only programme a pre-obese state in adult offspring, but also programmes feeding behaviour and anxiolysis when tested between 10 and 15 weeks of age( Reference Wright, Fone and Langley-Evans 26 , Reference Wright, Langley-Evans and Voigt 27 ). However, beyond programming of satiety regulation and anxiolysis, it remains unknown whether lactational exposure to the CD has an impact on non-spatial memory. Therefore, the present study explored the consequences of lactational CD feeding on recognition memory in adult offspring. Memory was tested in a novel object discrimination (NOD) paradigm. Originally devised by Ennaceur & Delacour( Reference Ennaceur and Delacour 12 ), the NOD procedure has been widely utilised to investigate the impact of genetic, physiological and pharmacological manipulations on recognition memory in rodents (for a review, see Dere et al. ( Reference Dere, Huston and De Souza Silva 28 )), and also proved sensitive to nutritional manipulations( Reference Kosari, Badoer and Nguyen 4 , Reference Jurdak and Kanarek 11 ). In contrast to the water-maze task, the NOD test does not involve high levels of stress or anxiety. In high-arousal memory tests, anxiolytic effects of hyperenergetic diets( Reference Murphy and Mercer 29 ) can contribute to diet-induced memory impairment( Reference Ross, Darling and Parent 30 ). Our previous finding that lactational CD feeding programmes anxiolytic effects in the offspring( Reference Wright, Langley-Evans and Voigt 27 ) would therefore preclude the aversive water-maze task as a test of choice. As direct exposure of rats to hyperenergetic diets has been reported to induce memory deficits, it was hypothesised that maternal exposure to CD feeding might induce a deficit in recognition memory in adult offspring.
Experimental procedures
Pregnant female Wistar rats (Harlan) were housed individually with ad libitum access to a standard laboratory chow (Teklad Global 18 %; Harlan) and water. The rats were maintained under a 12 h light–12 h dark cycle (lights on 08.00 hours), between 20 and 22°C. At birth, litters were reduced to four pups of each sex, and randomly allocated to either a standard laboratory chow diet (control) or the same chow diet in conjunction with the experimental CD. The latter consisted of a range of highly palatable human foods (pork pie, pate, cocktail sausages, cheese, crisps, jam, fruit and nut chocolate, golden syrup cake, shortbread and peanuts)( Reference Akyol, Langley-Evans and McMullen 31 ). Of these food items, four were provided daily and one of those was changed daily. At postnatal day 21, the offspring were weaned, group-housed with littermates of the same sex and maintained on the chow control diet for the remainder of the study.
For behavioural testing, a total of sixteen dams per feeding condition were used and eight pups from each litter were randomly allocated to a testing condition (n 10 per condition). Food consumption of the dams during lactation was closely monitored in an additional eight dams, four from each feeding condition. This was done in independent litters to avoid any possible handling-induced interference with behavioural testing. Energy intake (kJ) and macronutrient consumption (carbohydrates including sugar, fat and protein) were calculated from the manufacturers’ data. Weight loss due to evaporation was measured in triplicate samples of each individual food item placed in empty cages. The average daily percentage change in the weight of foods ranged from 0 to 6·2 % and corresponded to an average overestimation of energy intake by 2·51 % (7·5 kJ/d), which can be considered within an acceptable error of measurement( Reference Akyol, Langley-Evans and McMullen 31 ). Body weights of both dams and pups were measured at the beginning and the end of the study.
NOD testing was undertaken between 11 and 13 weeks of age, which is in the range of previous studies related to the subject( Reference Wright, Fone and Langley-Evans 26 ). For behavioural testing, ten pups of each sex were used. The methodology used in the present study was modified from King et al. ( Reference King, Sleight and Woolley 32 ). Briefly, the rats were habituated to the test arena (54 cm × 38 cm × 40 cm) in the absence of any objects for 1 h the day before testing. On the day of testing, the rats received an additional 3 min habituation session and were returned to the home cage for 1 min, before being placed into the observation arena for the training (familiarisation) trial with two identical objects for 3-min. In three independent experiments, each rat was then returned to the observation arena for 3 min for the test (choice) trial with one of the two objects replaced by a similar but novel object, either after a 1, 2 or 4 h inter-trial interval (ITI). The remaining object from the familiarisation trial was left untouched (familiar object). The objects were 150 ml water-filled plastic bottles with three horizontal stripes of either white or black 1·2 cm-wide masking tape being randomly assigned for each rat during the training schedule. The objects were positioned 13 cm from the length side and 11 cm from the width side of the arena in opposite corners. Arena and objects were cleaned with 70 % ethanol between the experiments to eliminate olfactory cues. During the two trials, exploration of each object (sniffing, licking, chewing or approaching the object otherwise at a distance < 1 cm) was recorded on a video and later analysed manually using Ethovision 3.1 (Noldus). Testing was undertaken in constant light (80 lux) between 08.30 and 15.00 hours.
The statistical unit for macronutrient and energy intake was the dam. Nutritional data and body weight of dams and pups were analysed using Student's t test. The statistical unit for behavioural testing was the pup. The study was powered to detect a difference of 40 % for the time spent in exploration, based upon σ = 4·8 (determined from published studies) and an α value of 0·5 at 80 % power. Object preferences during each NOD trial were assessed using three-way repeated-measures ANOVA (with object as the within-subject factor and diet and ITI as between-subject factors) applied separately to each sex and followed by Bonferroni's multiple-comparison post hoc test. Statistical analysis was conducted using SPSS 21 (IBM) and GraphPad Prism 6 (GraphPad). Values are expressed as means with their standard errors. P< 0·05 was regarded as statistically significant for all tests.
All procedures were performed under licence from the Home Office, in accordance with the Animals (Scientific Procedures) Act 1986 and after approval from the University of Nottingham Ethical Review Committee.
Results
Lactating CD-fed females had a higher energy intake due to overconsumption of fat and sucrose, although the overall carbohydrate intake was similar to the chow-fed controls. Protein intake was reduced in CD-fed dams (Table 1). Body weight as measured following parturition was similar in both groups (data not shown). The CD-fed dams gained more weight during lactation (29·8 (sem 1·3) g) than the chow-fed controls (17·8 (sem 2·2) g) (P< 0·01). By contrast, CD feeding did not have an impact on body weight in pups in the present study (data not shown) and in a previous study( Reference Wright, Fone and Langley-Evans 26 ).
Table 1 Daily energy and macronutrient intake in lactating dams (Mean values with their standard errors; n 4 dams per group as collected over 21 d of lactation)
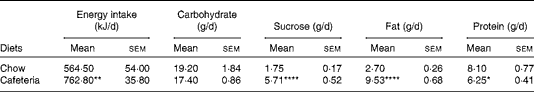
Mean value was significantly different from that of the chow-fed controls: * P< 0·05, ** P <0·01, **** P <0·0001 (Student's t test).
Neither male nor female offspring demonstrated any spatial preference for either identical object during the familiarisation trial, and there was no impact of the diet on the total levels of object exploration by either sex (data not shown).
After a 1 h ITI, male offspring were able to distinguish the novel from the familiar object, regardless of whether the dams received the chow diet (P< 0·001) or the CD (P< 0·0001) (Fig. 1(a)). After a 2 h ITI, male offspring from the CD-fed dams distinguished the novel from the familiar object (P< 0·01), but the controls showed no signs of memory, and neither group exhibited preferential exploration of the novel object after a 4 h ITI (Fig. 1(a)).

Fig. 1 Effect of maternal lactational diet on novel object discrimination in adult offspring following an inter-trial interval (ITI) of 1–4 h. Duration (s) spent by (a) males and (b) females exploring familiar (□) and novel (■) objects during the choice trial (n 8–10 per group). Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the familiar object in the same sex following the same maternal diet and ITI: * P< 0·05, ** P< 0·01, *** P< 0·001, **** P< 0·0001 (three-way repeated-measures ANOVA with Bonferroni's multiple-comparison post hoc test). CD, cafeteria diet.
Female offspring from the control dams successfully discriminated the novel object after an ITI of both 1 h (P< 0·05) and 2 h (P< 0·001), but in each case, discrimination was absent in female offspring from the CD-fed dams (Fig. 1(b)). However, there was a tendency in these female offspring from the CD-fed dams to discriminate the novel object after a 1 h ITI (P< 0·10). Taken together, these findings suggest that maternal exposure to the CD during lactation exerts a differential effect on cognitive performance in male and female offspring, with lactational CD exposure delaying memory decay in males and accelerating memory decay in females. Irrespective of maternal diet, neither sex showed any behavioural signs of memory after a 4 h interval.
Discussion
The present study tested the hypothesis that exposure to cafeteria feeding during the suckling period would have an impact on recognition memory in adult life. This was of interest given the previous observations that feeding and anxiety-related behaviours are targets for nutritional programming at this stage of life. Our findings confirmed that lactational CD feeding influenced the learning behaviour of Wistar rats.
The present study demonstrated that offering dams a CD during lactation led to an increased energy intake, largely due to overconsumption of fat and sucrose. We observed reduced protein intake, which has been reported in previous( Reference Akyol, Langley-Evans and McMullen 31 , Reference Warneke, Klaus and Fink 33 ), but not in all cafeteria, studies( Reference Esteve, Rafecas and Fernandezlopez 34 , Reference Llado, Pico and Palou 35 ). Although protein intake was significantly lowered by CD feeding, a 23 % reduction was not sufficient to have an impact on pup growth, suggesting that the protein deficit was modest compared with the overconsumption of energy, fat and sugars. We would rather suggest that programming and behavioural effects of diets mimicking a Western-type diet are complex and cannot be attributed to a nutritional imbalance of a single macronutrient.
Feeding of a hyperenergetic CD to rat dams during lactation had a significant impact on object recognition memory of the offspring in adult age. This finding provides further evidence that the lactational period is not only important for metabolic programming( Reference Akyol, McMullen and Langley-Evans 25 , Reference Daniel, Akyol and McMullen 36 ), but also for programming of behaviour, as we found both reduced anxiety and reduced behavioural satiety in parallel studies conducted under identical conditions( Reference Wright, Fone and Langley-Evans 26 , Reference Wright, Langley-Evans and Voigt 27 ).
The observed sex differences in chow-fed controls appear to be consistent with previous non-spatial NOD studies, where females proved to be superior to males, although the opposite is true for spatial versions of the test( Reference Sutcliffe, Marshall and Neill 37 , Reference Ghi, Orsetti and Gamalero 38 ). Although not controlled for in the present study, oestrogen (E2) has been shown to be associated with better NOD performance( Reference Walf, Rhodes and Frye 39 ) and could potentially modulate NOD through interactions with the brain serotonergic system (for a review, see McEwen et al. ( Reference McEwen, Akama and Spencer-Segal 40 )). Serotonin (5-hydroxytryptamine) plays a role in NOD (for a review, see Dere et al. ( Reference Dere, Huston and De Souza Silva 28 ); King et al. ( Reference King, Sleight and Woolley 32 )) and seems to be affected by early cafeteria feeding as we found in the hypothalamus of the offspring from CD-fed dams( Reference Wright, Fone and Langley-Evans 26 ). Therefore, 5-hydroxytryptamine–oestrogen interactions may account for the observed sex differences in the effect of early CD feeding programming on NOD, although an additional contribution of glucose levels is also possible.
In obese rats, fasting glucose levels are negatively correlated with NOD( Reference Jurdak and Kanarek 11 ). Although lactational CD feeding per se only predisposes the offspring to obesity and has a little impact on fasting glucose levels( Reference Akyol, McMullen and Langley-Evans 25 , Reference Wright, Langley-Evans and Voigt 27 , Reference Akyol, Langley-Evans and McMullen 31 ), male rats exposed to CD feeding in the lactation period show a more rapid glucose clearance in the blood following a glucose challenge, whereas in females, lactational chow leads to faster glucose clearance( Reference Akyol, McMullen and Langley-Evans 25 ). As exogenous glucose can enhance memory( Reference Gold 41 ) and brain glucose fluctuates depending on local demand( Reference McNay and Gold 42 ), it could be speculated that diet-programmed and sex-dependent differences in glucose metabolism/clearance could contribute to the differential effects of lactational cafeteria feeding on NOD learning in male and female offspring.
Maternal obesity, either due to high fat feeding or a sucrose-enriched diet, impaired reversal learning in the offspring, regardless of the type of hyperenergetic diet( Reference Wu, Deng and Li 13 ). The present study and other studies( Reference White, Pistell and Purpera 22 ) provide evidence that in rodents, an obesogenic environment in early life has an impact on cognitive functions in adult age. However, the precise outcome, either being positive or negative, depends on diet, memory model and possibly sex. In general, these rodent studies are relevant to the situation in humans where cognitive deficits have been attributed also to maternal obesity( Reference Van Lieshout 43 , Reference Sullivan, Nousen and Chamlou 44 ).
In conclusion, the present study shows that maternal exposure to CD feeding can programme NOD in adult life. In better-performing females, dietary programming interferes with NOD, whereas NOD was improved in males after actational CD feeding.
Acknowledgements
The authors gratefully acknowledge Asli Akyol, Carol Armett, Sarah Kirkland, Richard Plant and Karen Swift for their expert technical support.
The present study was supported by grant RSF5103 (to J.-P. W. V. and S. C. L.-E.) from the University of Nottingham. T. M. W. was supported by an IDTC studentship from the School of Veterinary Medicine and Science and the School of Biosciences, University of Nottingham.
The authors' contributions were as follows: J.-P. W. V. and S. C. L.-E. designed the experiment. T. M. W. and W. G. D. performed the experimental analyses, and collated the data. M. V. K. performed the statistical analyses. J.-P. W. V. wrote the manuscript. All authors discussed the results and commented on the manuscript.
There is no conflict of interest.






