Human milk (HM) is a food base for newborns; its proximate composition (water, fat, protein, carbohydrates and minerals) is balanced and contains unique substances, such as growth factors, antimicrobial factors, anti-inflammatory agents, digestive enzymes, micro-organisms and various types of hormones(Reference Taylor1). Some circumstances can hinder babies from accessing HM, such as premature births, difficulties suck milk, mothers with an infectious disease or low milk production volume. In many cases, the non-use of HM can drive malnutrition in the newborn, impair the development of different parts of the organism and, in later phases of life, led to the development of chronic diseases, immune deficiencies, obesity, attention deficit, diabetes and hypertension(Reference Victora, Adair and Fall2,Reference Cunnane, Francescutti and Brenna3) .
Rats fed with HM showed decreased lipid accumulation in skeletal muscle and inflammation status, improved insulin resistance and glucose disposal, and redox status increment. The literature also reports the influence of HM’s lipid profile on rat brain tissues(Reference Cavaliere, Trinchese and Musco4,Reference Aidoud, Delplanque and Baudry5) . It has been reported that sphingomyelin lipids (which contain high amounts of C16:1, C18:1n11 and long-chain fatty acids) were significantly higher in the brain tissues of recently weaned rats fed with HM or infant formula(Reference Su, Subbaraj and Fraser6). Hahn-Holbrook et al. (Reference Hahn-Holbrook, Fish and Glynn7) observed decreased sadness and distress in babies after HM intake, attributed to its high concentration of n-3 PUFA. The authors also linked HM cholesterol to brain maturation(Reference Hahn-Holbrook, Fish and Glynn7).
Moreover, according to Isaacs et al. (Reference Isaacs, Fischl and Quinn8), HM consumption affects white matter and cognition by increasing glial myelination. Therefore, ensuring the intake of HM by babies who have previously mentioned difficulties is a challenge to overcome. Human Milk Banks were created to contribute with alternatives to make it possible for babies to ingest milk, and they are responsible for actions aiming to promote, to protect and to support breast-feeding and for carrying out activities from a collection of nutritional tactics, selection, classification, use, quality control and HM distribution(9). However, in neonatal intensive care units, fat retention in nasogastric tubes is observed during newborns’ feeding by infusion pumps(Reference Stocks, Davies and Allen10). Hence, actions must be adopted to minimise fat retention and deliver the food full-energy load to newborns.
In this context, homogenisation is a unit operation incorporated in HM processing to modify the milk flow characteristic and diminish fat retention in the tubes. Homogenisation can decrease the fat globules’ size, contributing to increased HM stability and easing its flow(Reference García-lara, Escuder-vieco and Díaz11–Reference Claire and Thomaz13). Hence, milk homogenisation is expected to promote a reduction in holding nutrients in probes. Consequently, the newborns can absorb a higher fraction of the energy content present in milk ingested. Homogenisation (a) supplies energy to the milk system, (b) promotes the fat globule’s rupture, (c) reduces the fat globules’ average size and (d) contributes to increasing the system’s configurational entropy. This increase in configurational entropy slows down the phenomenon of coalescence. Variations in Gibbs energy in a system indicate the most likely state thermodynamically. The phenomenon occurs even though the conversion is kinetically very slow(Reference McClements14). The shear rate during milk homogenisation favours the rupture of the fat globule membrane. Thus, many droplets are formed at both a smaller average diameter and a higher number than the native ones, leading to an increase in the total interfacial area. Proteins with surfactant properties diffuse to the interface since the number of phospholipids naturally present in the fat globule interfaces is insufficient to cover the new expanded interfacial area. The interfacial tension reduces, and the colloidal system tends to achieve some kinetic stability(Reference McClements14). The coalescence effect can be avoided if (i) the energy supplied to the system is enough to overcome the interaction force between the droplets, (ii) there is enough surfactant to reduce the Gibbs–Marangoni effects(Reference McClements14).
Thereby, the homogenisation may be connected to the ingestion of an improved range of nutrients and can influence different metabolic processes in the newborn’s body. The in vivo study can also identify the effects of homogenised HM consumption on the newborn’s metabolism and demonstrate the homogenisation stage’s safety in HM processing(Reference Correa15,Reference Rayol, Martinez and Jorge16) . Therefore, in the present work, HM was used as a supplement in the diet of male Wistar rats to determine: (i) the body gain and weight metrics of animals, (ii) the influence of the homogenised HM intake on the biochemical profile of the animal blood, (iii) the lipid profile of long-chain fatty acids in the animal brain and (iv) the effect of the ingestion of homogenised HM on the morphofunctional aspects of the liver, intestine and adipose tissue of animals. This work is relevant because it highlights the addition of the homogenisation stage in HM processing and its influence on Wistar rats’ nutrition.
Methods
Ethical statement
The experiment with rats followed the European Union Regulation on the Care and Use of Laboratory Animals (OJ L 358; 18·12·1986) and its associated guidelines, the EU Directive 2010/63/EU for animal experiments and the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). The procedure was approved by the Animal Use Ethics Committee of the Universidade Federal de Viçosa, according to protocol number 66/2018.
Obtaining, processing and characterisation of pooled human milk
Pooled HM used in the experiments was characterised and classified according to the criteria of the Brazilian National Network of Human Milk Banks at the São Sebastião Hospital. All the material was stored in glass bottles, according to Brazilian regulation(9). HM from the three stages of lactation, that is, colostrum, transition milk and mature milk, was used at appropriate microbiological conditions. Samples containing only hair or skin were considered unsuitable for processing in the Human Milk Bank and, hence, discarded. Such milk was then donated to be used in our experiments. Thus, all donated milk was mixed to form pooled crude HM, filtered (Whatman 1–125 mm qualitative paper, 45 µm) and fractionated in three portions of 1 litre each. The schematic diagrams of the processing lines of pooled crude HM are presented in Fig. 1. Processing line 1 represents the pasteurised human milk (PHM) production following the standard process adopted in the Brazilian banks of HM(9). Line 2 corresponds to the processing of pasteurised and homogenised human milk (PHHM), in which pasteurised milk was homogenised using a mixing processor (Walita) at 4800 rpm for 3 min. In processing 3 (pasteurised and skimmed human milk, PSHM), HM was centrifuged at two cycles of 13 000 g for 10 min, next pasteurised and then used as the negative control to evaluate the effect of fat in milk processing.
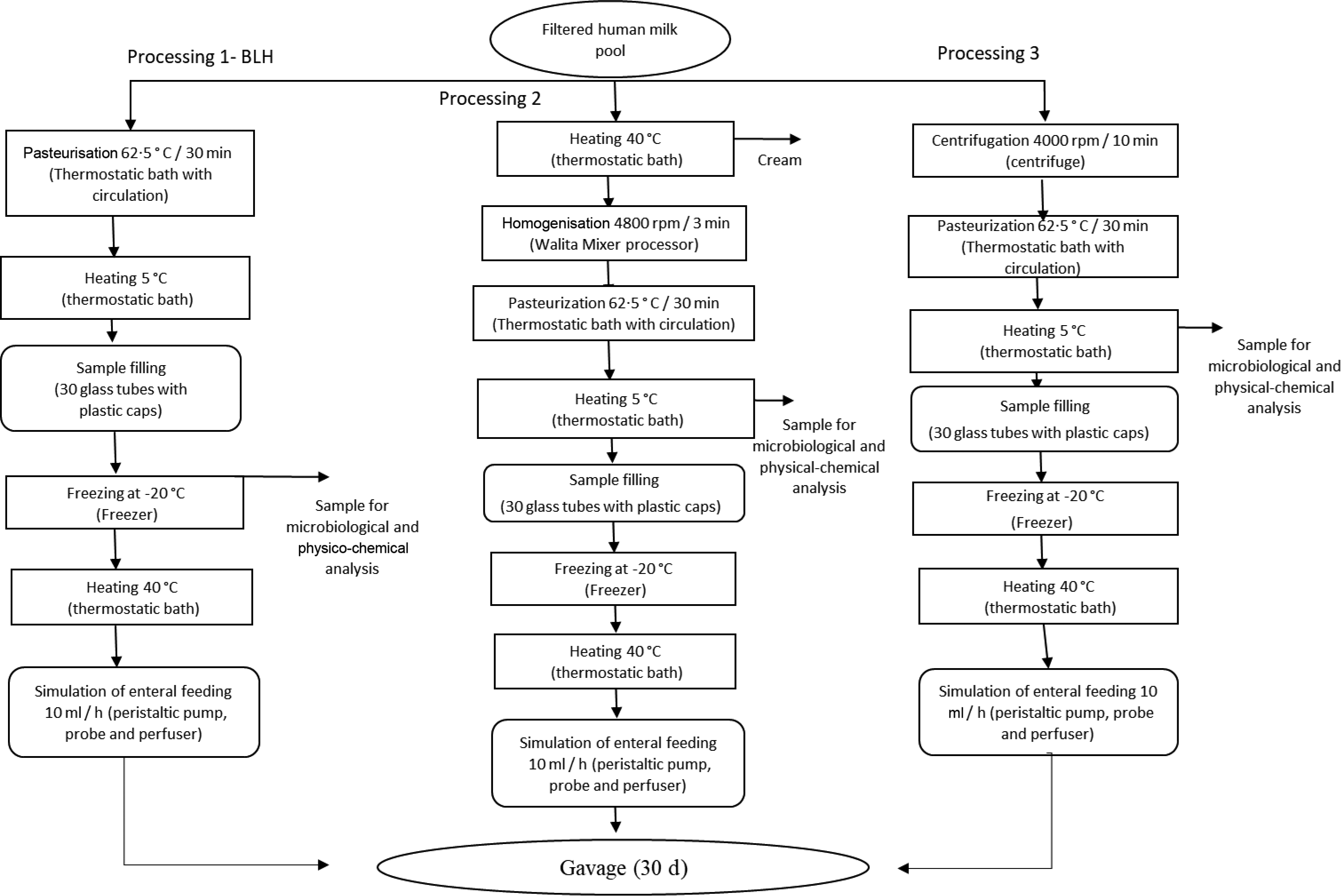
Fig. 1. Human milk (HM) processing flow chart. Process 1: HM processed according to the Human Milk Bank (HMB). Process 2: HMB processing added of the homogenisation step. Process 3: Skimmed human milk processing.
After each processing, each type of produced pooled HM was characterised in terms of pH, titratable acidity, protein content (Kjeldahl method), fat content (Gerber method), total dry extract, fixed mineral residue and total coliforms(17). The proximate composition of each type of HM was determined before and after milk flowing through the prototype used for animal feeding. Such a prototype simulates a unit of the neonatal enteral feeding.
The prototype used for animal feeding
The prototype, similar to the devices used in neonatal units for enteral feeding of babies, was utilised to evaluate fat retention inside the nasogastric tubes. One consists of an infusion pump (B. Braun Melsungen AG), a perfuser and a 100 cm probe polyvinyl chloride (Mark Med®). The flow rate of milk was 10 ml/h.
Animals, housing and husbandry
Thirty-two male Wistar rats (Rattus norvegicus albinus) with 21 d of age were used. The animals came from the Central Vivarium of the Center for Biological and Health Sciences/Universidade Federal de Viçosa and were accommodated in individual cages. The rats were placed in a room maintained at 22 (sd 1) °C and a light-dark cycle of 12 h for 30 d.
Study design
The thirty-two animals were randomly schematised into four groups (n 8/group), composed of two control and two test groups. The animals received, daily, via gavage: water (control group); PSHM (negative control group); PHHM (test1 group); and PHM (test2 group). All groups were given access to commercial diets and water ad libitum during the 30 d of the experiment.
Experimental procedures
The weekly volume of HM offered to the rats for the weight gain was within the recommended amount range between 1 and 2 ml/100 g of body mass(Reference Meier, Engstrom and Patel18) to avoid animal stress or discomfort. Consequently, the group’s average body mass with the lowest average weight (weekly) was used to calculate HM’s volume to be administered in the following weekly. The volumes of each HM type offered by gavage were 0·86 ml (week 1), 1·3 ml (week 2), 1·6 ml (week 3) and 2 ml (week 4), totaling 43 ml/rat per 30 d.
Sample size
The Student’s t-test was used to calculate the number of repetitions. No previous experiment was performed to calculate the ideal number of repetitions. Thus, de Almeida et al. (Reference de Almeida, de Queiroz and Ribeiro de Queiroz19) procedure to estimate the variance and minimum difference between means was used. These authors assessed the effectiveness of food consumption from different lipid sources, calculated as the ratio between weight gain and total food consumed. The number of 8·07 repetitions was determined using four treatments, at a significance level of 5 %. Such a number was close to that suggested for eight repetitions. Thus, for our experiment, thirty-two animals were requested. That is a total of eight repetitions per treatment. Online Supplementary Appendix 1 shows the calculation of the ideal number of repetitions.
Experimental outcomes
Body weight
The animals’ body weight was recorded before the first dose (day 1) and twice a week until the end of the study. Food consumption was measured weekly. The resulting data were used to calculate the feed efficiency ratio (FER), defined as the relationship between animal weight gain and dietary intake:
Body metrics (abdominal and thoracic perimeters) were determined at the beginning and the end of the diet. The difference between the body metric values at the beginning and the end of the experimental period was calculated. The (abdominal perimeter)/(thoracic perimeter) ratio was used to calculate the BMI and Lee’s index.
Biochemical analyses
At the end of the experiment (30 d), the animals were fasted (12 h) and submitted to euthanasia by cardiac puncture after anaesthesia with 100 % isoflurane (Isoforine, Cristália®). The blood was collected in heparinised tubes (13 × 100 mm; BD Vacutainer®) and centrifuged (Fanem-204) under refrigeration (4 °C) for 10 min at 1600 g to obtain plasma. The plasma was separated and stored at –80°C for biochemical analyses. Total cholesterol, HDL-cholesterol, TAG, alanine aminotransferase and aspartate aminotransferase were determined using commercial kits (Bioclin®) and a spectrophotometer (Mindray Medical International Limited, model BS 200)(Reference e Dias, Siqueira and da Conceição20).
Assessment of liver tissue
Hepatic lipid was extracted using organic solvents(Reference Folch, Lees and Sloane Stanley21), and its content was determined by a colorimetric method in a microplate spectrophotometer (Multiskan Sky, Thermo Fisher Scientific)(Reference Rosa, Grześkowiak and Ferreira22).
Profile of unsaturated fatty acid and SFA in rat brain tissues
The brain’s total lipid content was converted to fatty acid methyl esters to obtain its fatty acid profile. This analysis was carried out in a GC equipped with a flame ionisation detector (Shimadzu, GC-2010)(Reference da Silva, de Paula Correa and Martins23).
Histological analysis and microtomography of the femur
Histological analysis. Fragments of the liver and small intestine of the animals were removed and fixed, for 24 h, in Carson’s formalin at room temperature. After fixation, the tissues were dehydrated in solutions of an increasing gradient of ethanol (70 % to absolute alcohol) and then included in hydroxymethyl methacrylate resin (Historesin, Leica®). Cross-sectional and longitudinal sections of 3 µm thickness were obtained using a rotating microtome (RM2155, Leica®) and next stained in haematoxylin–eosin solution. Images were captured with a 20× objective under a light microscope (Primo Star 2012, Zeiss®) using an Aixo ERc5s video camera (Zeiss®) to carry out the morphometric analysis of the liver and small intestine. For the small intestine, the villus height and width (apical, middle and basal region) and depth of the Lieberkühn crypts were measured with the aid of the Image J® software version 4.5 (Media Cybernetics)(Reference e Dias, Siqueira and da Conceição24).
The fragments of abdominal adipose tissue were dehydrated, embedded in paraffin (Paraplast Plus, Sigma®), cut to 5 µm thickness and stained with haematoxylin and eosin. The lamina was assembled with the aid of Entellan (Merck®). Ten fields per animal were captured to measure adipocytes’ area, directly from the light microscope (Primo Star 2012, Zeiss®) with a 20× objective(Reference e Dias, Siqueira and da Conceição20). The average values obtained from the intestine and adipose tissue were analysed by the Tukey test at the level of 5 % probability. Microstructural characterisation was made using a SkyScan high-resolution X-ray microtomography (model 1174v2). The measurements obtained were trabecular volume (BV/TV), trabecular thickness (Tb/Th), number of trabeculae (Tb·N) and thickening between trabeculae (Tb·Sp)(Reference Aljewicz, Tonska and Juskiewicz25).
Statistical analyses
The Kolmogorov–Smirnov test was used to determine the data normality. After confirming normality, the data were subjected to the ANOVA followed by the Tukey test at 5 % probability (P < 0·05). The analyses were performed using the SAS statistical software, version 9.2.
Results and discussion
Human milk characterisation
Table 2 shows the average composition of the three types of processed pooled HM (PSHM, PHM and PHHM) before and after flowing through the prototype probes. A decrease in the total dry extract, fat, carbohydrate and fixed mineral residue after flowing was documented in the PHM. PSHM exhibited a decrease in the contents of total dry extract and carbohydrates. (PHHM did not show a composition change after flowing through the prototype and presented 3·14 % of fat, 2·04 % of protein and 5·79 % of lactose. Such contents are similar to those in the pooled crude HM; therefore, pasteurisation and homogenisation of HM did not significantly change the HM composition (P < 0·05).
Table 2 reveals the nutrient holding inside the infusion pump probes after PHHM flowing. Fat and some fat-soluble components tend to adhere to the wall of nasogastric tubes during the milk flow through the probe, causing imbalances in the newborn’s nutrition. Thus, the addition of the homogenisation step in milk processing promoted a reduction in the milk fat globules’ diameter, more structural stability, better milk flow, and consequently, the newborn would receive a higher quantity of total solids(Reference García-lara, Escuder-vieco and Díaz11,Reference Rayyan, Rommel and Allegaert26) . Milk fat affords essential fatty acids that compose cell membranes’ structure, absorb fat-soluble vitamins and construct hormones(Reference Milner and Allison27,Reference Mehrotra, Sehgal and Bangale28) .
Pooled crude HM was pasteurised at 65°C/30 min, which is a sufficient process condition to destroy pathogenic micro-organisms and reduce the rate of spoilage. The number of coliforms (at 35 and 45°C) in PHM was inferior to 3·0 most probable number of coliforms (MPN/g) within the expected range of the Brazilian regulation criteria(9). Hence, PHHM would guarantee milk supply with a higher concentration of nutrients at the end of the infusion process. The addition of the homogenisation step in the processing line of HM is also made possible.
Feed intake, body mass and animal metrics
The impacts of feed intake on weight gain and FER are shown in Fig. 2. Since FER is associated with the animal’s weight gain and the food consumed, Fig. 2(A) shows the mass body gain during 4 weeks, and Fig. 2(B) the feed intake in 4 weeks. There was observed no significant difference (P < 0·05) for the relative weight of the organs and body metrics (Table 1).

Fig. 2. (A) Rat weight gain according to the following weeks. (B) Average feed consumption per week. (C) Feed efficiency ratio (FER). Treatments: PHM, pasteurised human milk; PHHM, pasteurised–homogenised human milk; PSHM, pasteurised–skimmed human milk. n 8 animals/group. Values are expressed as mean values and standard deviations. Bars with the same letters showed no significant difference from the Tukey test (P < 0·05). ![]() , control;
, control; ![]() , PHM;
, PHM; ![]() , PHHM;
, PHHM; ![]() , PSHM.
, PSHM.
Table 1. Average values of body metrics and relative weight of organs*
(Mean values and their standard deviations)

* n8 animals/group: samples with a normal distribution. Different letters on the same line indicate statistical difference (P < 0·05) between groups, using the ANOVA test followed by the Tukey test.
The animals supplemented with diets containing PHHM exhibited a weekly lower fed consumption than the other groups (P > 0·05). At the last week of the in vivo tests, the PHHM group animals showed low weight gain (P > 0·05). Despite the lower feed intake, the animals of the PHHM group remained healthy and developed as expected.
Thus, HM processing may not affect the mass body gain, even if the feed consumption is low because FER did not significantly differ among the groups (Fig. 2(C)). Such behaviour may be due to the good digestibility and absorption of milk nutrients, which promoted the animals’ mass body gain.
Breast milk is rich in long-chain fatty acids that act, for example, in human satiety. Thus, the rich composition of HM in fatty acids(Reference Barreiro, Díaz-Bao and Cepeda29–Reference Nishimura, de Castro and Jordão Junior31) can be connected with satiety because some fatty acids help absorb nutrients in the intestine(Reference Havlicekova, Jesenak and Banovcin32). However, the reason is not exact. The sn-2 bond acts in digestion control and subsequent fat absorption. Among the fatty acids of HM, palmitic acid, for example, represents 20–25 % of the total fatty acids of HM. Nearly 70 % of palmitic acid composition corresponds to TAG, which are esterified in the sn-2 position. As palmitic acid absorption slows intestinal motility, the intestine may have time for the occurrence of all reactions, leading the animal to greater satiety(Reference Innis, Dyer and Nelson33). Besides, fat globules of homogenised human milk are smaller(Reference Zhao, Du and Mao34) and, consequently, have a larger surface area. The increase of surface area makes the enzymes’ performance and lipids’ digestion easy, helping with the absorption(Reference Singh, Ye and Horne35).
Regarding a link between satiety and the other nutrients, HM contains bioactive proteins, helping the intestine’s nutrient absorption. The α-lactalbumin protein, for example, may increase the absorption of Fe and Zn and provide essential amino acids(Reference Kelleher, Chatterton and Nielsen36,Reference Ali37) . In a smaller number, phosphorylated peptides may act in the absorption of Ca+2(Reference Kang, Jhoo and Pak38). Most HM proteins are absorbed quickly by the body. Breast milk also contains human milk oligosaccharides, which (a) favour more excellent absorption of the nutrients ingested(Reference Wu, Johnson-Henry and Sherman39); (b) act as prebiotics, promoting beneficial intestinal bacteria’s growth and generating SCFA, essential for intestinal health(Reference Cacho and Lawrence40); (c) modulate the host’s epithelial immune responses and (d) reduce the binding of pathogenic bacteria and viruses selectively to the intestinal epithelium, preventing the onset of diseases that can disrupt the absorption of nutrients by the intestine(Reference Walsh, Lane and van Sinderen41).
Studies have shown that diets supplemented with HM decrease the inflammation state of mitochondrial cells(Reference Trinchese, Cavaliere and Canani42), improve glucose elimination and insulin resistance and reduce lipids accumulation in skeletal muscle(Reference Cavaliere, Trinchese and Musco4,Reference Trinchese, Cavaliere and De Filippo43) .
In the last week of the in vivo experiments, the animals ingested (per gavage) a higher volume (2 ml/d) of HM than in the three preceding weeks. Thus, diet ingestion increments may have contributed to more excellent absorption of nutrients, bringing satiety since milk had a high-fat content. We cannot rule out the possibility of animal stress because it could result in lower feed consumption.
Biochemical assays
Table 3 shows the effects of HM process conditions on the rats’ biochemical indices (cholesterol, HDL and TAG of animal’s blood), referring to the four groups studied (controlb; PHMb: pasteurised human milk; PHHMb: pasteurised–homogenised human milk and PSHMb: pasteurised–skimmed human milk). There was no significant difference among groups for cholesterol, HDL and TAG data. The results observed in Table 3 agree with those found in the literature for Wistar rats at the same age. According to the range cited by Ali et al. (Reference Ali37), the values found in our work for total cholesterol (92·20 (sd 14·24) mg/dl), HDL (35·00 (sd 2·34) mg/dl) and TAG (51·40 (sd 10·84) mg/dl) are representative of a healthy animal(Reference Ali37).
Table 2. Composition and process conditions of human milk (pooled crude; PHM, pasteurised; PHHM, pasteurised–homogenised; PSHM, pasteurised–skimmed) before and after flowing through the probes of the infusion pump prototype*
(Mean values and their standard deviations)

* All analyses were performed in three repetitions. Four experiments/twelve samples. Different letters on the same line indicate statistical difference by the Tukey test (P < 0·05).
Table 3. Biochemical profile of plasma of Wistar rats fed with water and human milk by gavage. Diet of rat groups: controlb, water; PHMb, pasteurised human milk; PHHMb, homogenised–pasteurised human milk; PSHMb, skimmed–pasteurised human milk*
(Mean values and their standard deviations)

* All analyses were performed in eight repetitions/group. Four treatments/thirty-two samples. Equal letters on the same line indicate statistical equality by the Tukey test (P < 0·05).
High values of cholesterol and TAG indicate a propensity for higher adiposity rates. The study of total cholesterol can predict ischaemic heart disease, while TAG in high concentrations indicate the risk of CVD(Reference Lemieux, Lamarche and Couillard44). The present results indicate no difference in total cholesterol and TAG.
Regarding the analysed enzymes (alanine aminotransferase and aspartate aminotransferase), there was no significant difference between groups (P < 0·05). The average values of alanine aminotransferase (150 U/l) and aspartate aminotransferase (35 U/l) found in our experiments are in the range reported for a healthy animal(Reference Kang, Jhoo and Pak38).
The values of total lipids, cholesterol and TAG showed no difference for the liver (P < 0·05). Thus, the biochemical assay results allowed us to infer that the rat’s diet supplemented with HM kept the biochemical variables in the normal ranges.
Fatty acid profile of human milk
Table 4 shows the fatty acid profile of studied HM (PSHM, PHM and PHHM) compared with the literature data on fatty acid compositions of HM.
Table 4. Fatty acid composition (%) of human milk (PHM, pasteurised; PHHM, pasteurised–homogenised; PSHM, pasteurised–skimmed) after flowing through the probes of the infusion pump prototype*
(Mean values and their standard deviations)

* Normal distribution of fatty acids. Different letters on the same line indicate statistical difference by Tukey test (P < 0·05).
All fatty acids quantified in the present work exhibited concentrations in the range reported by Perrin et al. (Reference Perrin, Pawlak and Dean45), as shown in Table 4. The higher concentration of C8:0, C16:0, C18:0, C21:0, C16:1, C18:1n9c, C18:3n3; C24:1n9, C22:6n3c C18:2n6c and C20:4n6 was verified in PHHM compared with pooled crude HM. The difference in the concentration of fatty acids among the types of studied milk can probably be due to the fat retention in the probes used to feed the animals(Reference García-lara, Escuder-vieco and Díaz11,Reference Martinez, Desai and Davidson12,Reference Correa15) .
Among the fatty acids found in the three types of HM(Reference Perrin, Pawlak and Dean45), the essential ones stand out, long-chain precursors linoleic acid and α-linolenic acid and the long-chain fatty acids (arachidonic acid, EPA and DHA). Such fatty acids can act in antidepressant and cognitive mechanisms. The absence of some fatty acids (n-3), such as DHA, impairs spatial learning and memory in rodents. Studies have shown that the level of DHA depends almost entirely on food, and the endogenous synthesis of DHA from α-linolenic acid is limited in mammals(Reference Brouwer-Brolsma, van de Rest and Godschalk46–Reference Innis49).
Fatty acid profile of brain tissues of Wistar rats
The fatty acid profile of the animals’ brain tissues is shown in Table 5. There is a difference in the concentration of C20:4n6, C22:6n3 and C24:1n9 (P > 0·05) that is higher for the group fed with PHHM. This PHHM group also showed a more significant fraction (%) of DHA and nerve acid over the other groups (P < 0·05). All values are within the literature range, reinforcing the contribution of the homogenisation stage to the better use of nutrients available in HM. The PHHM group also exhibited an average concentration of C21:0 fatty acid higher than the values of literature and other evaluated groups. The influence of maize intake on the concentration of C21:0 fatty acid in bovine milk was already reported(Reference Medeiros da Silva, Restle and Luis Missio50).
Table 5. Profile of fatty acids (%) present in the brain tissue of the rats. PHMc, pasteurised human milk; PHHMc, pasteurised–homogenised human milk; PSHMc, pasteurised–skimmed human milk*
(Mean values and their standard deviations)

* n 8 animals/group. Different letters on the same line indicate statistical difference by Tukey test (P < 0·05).
The reduction in α-linolenic fatty acid content can be explained based on its action as a precursor to DHA and EPA found in high concentrations in HM(Reference Gerster51). DHA promotes brain and visual development, reduces cardiovascular morbidity and mortality, fights inflammatory conditions, fights cognitive decline and helps prevent cancer(Reference Balakrishnan, Dhavamani and Sadasivam52).
More significant variations were not observed in the brain’s fatty acid profile of animals, probably due to the small volume of HM in the diet. Despite such a reduced amount of milk, some fatty acids were found in higher concentrations in PHHM than in the other groups.
Histology of liver, adipose tissue and intestine
The liver’s photomicrographs, intra-abdominal adipose tissue and intestine are shown in Fig. 3 and Table 6. Liver histology can help identify diet changes and damage health as the liver is an organ responsible for metabolising and storing nutrients. The liver histology results (Fig. 3) showed no degeneration, liver inflammation and necrosis for any of the groups analysed. The healthy appearance of the central vein was evident.
Table 6. Statistical analysis of the histological assessments. Area of intra-abdominal adipose tissue and crypts number of intestine of animals fed with pasteurised human milk (PHMc), pasteurised–homogenised human milk (PHHMc, and pasteurised–skimmed human milk (PSHMc)
(Mean values and their standard deviations)

Different letters on the same line indicate statistical difference by Tukey test (P < 0·05).

Fig. 3. Effect of processed human milk intake on (A1) liver tissue architecture: (A) control group, (B) PHM, (C) PHHM, (D) PSHM, (CV) central vein, ×20 magnification; (A2) Adipocytes in intra-abdominal adipose tissue: (E) control group, (F) PHM, (G) PHHM, (H) PSHM, 20× magnification; (A3) Intestine tissue: (I) control group, (J) PHM, (K) PHHM, (L) PSHM, 10× magnification. (C) Crypts. n 8 animals/group. Twenty images of each coloration per animal were captured directly from the light microscope (Zeiss®, Primo Star model) through a photographic camera (Zeiss®, Aixo ERc5s model).
The ratio number of adipocytes/area of the adipose tissue showed a significant difference between the groups (Fig. 3) by the Tukey test at the 5 % level of significance. In the common area of the adipocytes, high averages were verified for the control group (7581 (sd 1417) µm2), PHM group (7162 (sd 1765) µm2) and PSHM group (5528 (sd 1853) µm2). The mean value of 4010 (sd 1898) µm2 was determined for the PHHM group. Blázquez-Medela et al. (Reference Blázquez-Medela, Jumabay and Rajbhandari53) found approximately 5500 µm2 as the average area of adipocytes, which corroborates our findings. Adipocytes are essential to be studied, as their metabolism affects body fat accumulation(Reference Orci, Cook and Ravazzola54,Reference Zhang, Guan and Townsend55) . Besides, smaller adipocytes with a smaller area reduce the chances of rapid weight gain and obesity(Reference Gao, Jia and Yang56). The adipose tissue results indicate that rat gavage feeding using PHHM can reduce the area and the adipocyte’s number.
The small intestine analysis reveals no difference in the intestinal villi’s number, height and width. Studies reported a direct correlation among the number, height and width of the intestinal villi with the mother’s diet during pregnancy and the first days of breast-feeding(Reference Buccigrossi, De Marco and Bruzzese57).
On the other hand, the crypts’ depths showed a significant difference (P < 0·05) among groups. The PHHM group exhibited a higher average than the PHM group. The crypt compartment has components that produce functional cells(Reference Itzkovitz, Blat and Jacks58). Therefore, the ingested nutrients’ profile is essential for the development and activity of the small intestine. Some milk constituents, such as lactoferrin, facilitate the baby’s intestinal adaptation by developing absorption cells(Reference Buccigrossi, De Marco and Bruzzese57).
Microtomography analysis of the femur
The porosity of bone tissue is divided into cortical (compact, 10 %) and trabecular (spongy, 90 %). These proportions may vary because the trabecular part can be associated with the mineral deficiency, that is, the thicker the trabecular part, the smaller the mineral mass(Reference O’Neill, Stutz and Mignemi59).
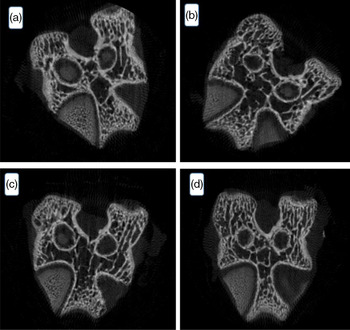
The result of the microtomography of the rat’s femur is seen in Fig. 4. The variables tissue volume, trabecular thickness, trabecular number and trabecular separation did not differ among groups (P > 0·05). Despite the lower feed intake by the animals of the homogenised HM group, there was no bone tissue difference among the groups. Data of statistical equality show the contribution of HM intake to the ingestion of minerals and corroborate the FER value determined in the present work.

Fig. 4. Effect of processed human milk intake on the trabecular bone cut: (A) control group, (B) PHM, (C) PHHM, (D) PSHM. n 8 animals/group. A hundred images per animal were captured.
Conclusions
Reducing fat content in non-homogenised human milk suggests that homogenisation is crucial to minimise milk nutrients’ retention in the enteral diet prototype’s probes. The supplementation of Wistar rats’ diet with HM did not affect the blood biochemical parameters nor the FER value. Such findings point out an adequate conversion of the food ingested into the mass gain. Histological analyses of the intestine from the animals fed with PHHM exhibited a healthy liver and an increment in the crypts’ depth. The adipocyte count showed a difference between the rat groups treated with PHHM and the other groups. The fatty acid profile of the animal brain tissue of the PHHM group changed after the gavage feeding, increasing the content of some essential fatty acids in brain tissue. The microtomography of the femur showed healthy bone tissues for all groups analysed. The homogenisation of HM provided benefits to Wistar rats’ diet and preserved the animals’ health. Therefore, the homogenisation stage in the process line of HM can be considered a safe and promising approach to enhance the quality of milk in Human Milk Bank.
Acknowledgements
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). The authors are grateful for the technical assistance of the team of Human Milk Bank from the Hospital São Sebastião, Viçosa, MG, Brazil during the accomplishment of this work.
K. de P. C.: conceptualisation, designing the study, methodology, data collection, data analysis, visualisation, writing – first draft preparation, writing – reviewing and editing; M. E. T. da S.: designing the study, data analysis, finding interpretation, reviewing – first draft preparation, writing – reviewing and editing; O. A. S. R.: designing the study, data analysis and finding interpretation; S. L. P. da M.: conceptualisation, resources, designing the study and methodology; M. do C. G. P.: conceptualisation, resources, designing the study, methodology and finding interpretation; Eduardo B. de Oliveira: resources and writing – reviewing; J. S. dos R. C.: conceptualisation, funding acquisition, resources, supervision, designing the study, methodology, reviewing – first draft preparation, writing – reviewing and editing. All authors read and approved the final manuscript.
No conflict of interest could be perceived as prejudicing the impartiality of the research reported.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114521001380