Patients with chronic kidney disease (CKD) have an increased risk of hospitalisation and mortality. This is especially so for patients with end-stage renal disease (ESRD)(Reference Wang, Yang and Long1). According to the 2018 annual report of the Renal Data System of the USA, nearly two-thirds (63 %) of all existing patients of ESRD were receiving haemodialysis therapy. This number will keep rising due to an increase in the population of existing ESRD patients(Reference Saran, Robinson and Abbott2). Over the years, the survival of patients on dialysis has improved. However, only 57 % of haemodialysis patients survive 3 years after the onset of ESRD. Notably, almost half of the deaths are related to CVD(Reference Saran, Robinson and Abbott2,Reference Thomas, Wulf and Bikbov3) . This high mortality provided an impetus to identify more modifiable risk factors to improve the survival of dialysis-dependent patients.
Dietary fibre comprises edible plant part of the diet that is difficult to digest and cannot be absorbed in the human small intestines(Reference Turner and Lupton4). Dietary fibre is considered to be an important component of a healthy diet because it interacts with the gastrointestinal tract and positively affects the digestive system. Considerable epidemiological evidence has revealed that high dietary fibre intake (DFI) reduces the risk of several chronic diseases such as CVD, type 2 diabetes, cancer, obesity and CKD(Reference Anderson, Baird and Davis5,Reference Mirmiran, Yuzbashian and Asghari6) . Moreover, an inverse association between dietary fibre and mortality was reported in general and CKD populations(Reference Krishnamurthy, Wei and Baird7,Reference Threapleton, Greenwood and Evans8) . It is noteworthy that dialysis population is special among CKD patients. This is because dialysis patients have a higher mortality rate and stricter dietary restriction compared with patients with other stages of CKD. Sometimes these patients have reverse epidemiological phenomena. There is a need to explore the effect of dietary fibre on mortality in dialysis patients. Notably, data on the association between dietary fibre and mortality, especially CVD mortality, in dialysis population are limited.
To address this gap in knowledge, the associations between DFI and all-cause and CVD mortality were evaluated. Potential effect modifications in patients undergoing maintenance haemodialysis (MHD) was assessed using data from a multicentre prospective cohort study in China.
Method
Study design and participants
The design of this study and some of the results have been reported previously(Reference Yang, Qin and Li9,Reference Chen, Qin and Li10) . Briefly, this was a multicentre cohort study conducted prospectively from January 2014 to December 2015 in eight outpatient dialysis centres, namely Nanfang Hospital, The First People’s Hospital of Foshan, Huadu District People’s Hospital of Guangzhou, Guangzhou Red Cross Hospital, Jinan University First Affiliated Hospital, The Third Affiliated Hospital of Southern Medical University, Southern Medical University Affiliated Nanhai Hospital and Shenzhen Second People’s Hospital. These centres are located in Guangdong province in China.
The inclusion criteria for participants were ≥18 years, undergone MHD for a minimum of 90 d (prevalent haemodialysis patients) and had a normal intake (not receiving enteral or parenteral nutrition). The exclusion criteria included history of physician-diagnosed severe infection, serious gastrointestinal disease, hyperthyroidism, liver cirrhosis, multiple organ failure, cognitive disorder or malignant tumour at an advanced stage according to participants’ previous medical records, because these conditions may influence dietary nutrient intake.
Overall, the information of 1302 patients was collected and recorded in this cohort. However, we did not have a detailed number of cases for patients who were excluded due to the exclusion criteria. Furthermore, a total of 1044 patients were included in the final analysis, after further excluding participants who enrolled in the cohort twice (n 2), received peritoneal dialysis (n 8), haemodialysis duration <3 months (n 163) and lacked baseline biochemical parameters and Kt:V ratio data (n 85).
Possible events of endpoint and vital signs of participants were recorded by physicians and trained research staff during each follow-up visit. This study was approved by the medical ethics committee of Nanfang Hospital, and all participants signed informed consent before participating in the study.
Data collection and measurements
Demographic data were collected by trained research staff as per standard operating procedures. Data on age, sex, co-morbidities (diabetes mellitus, hypertension, history of CVD), education level, smoking status, alcohol consumption and duration of dialysis were collected. Each participant was interviewed using a standardised questionnaire specifically designed for this study. Here the definition of diabetes mellitus was a past medical history of diabetes or the use of glucose-lowering medication, while that of hypertension was having a medical record of hypertension or using anti-hypertensive drugs. A history of CVD was defined as a previous history of myocardial infarction, congestive heart failure, angina, transient ischaemic attack or cerebrovascular accident and peripheral arterial disease. Patients who had a recent history of smoking or drinking were defined as having smoking status or alcohol consumption, respectively.
Physical examinations were carried out after dialysis when the dry weight of the patient could be estimated. The measurement of BMI was done using the formula: BMI = weight/height squared (kg/m2).
Blood samples were obtained from patients prior to an haemodialysis session in mid-week at baseline. Biochemical parameters, including serum albumin, Ca, phosphate, creatinine, TAG and C-reactive protein (CRP), were determined at each local dialysis centre as per the standard protocol. Total Kt:V ratio was calculated using the urea kinetic modelling (UKM) formula:
where UF is ultrafiltration, BUN is serum blood urea nitrogen and t is effective dialysis time.
Dietary intakes were registered by trained interviewers using a 24-h dietary recall that is known as the automated multiple pass method. Data were collected on 3 d (2 non-dialysis days and 1 dialysis day) in 7 consecutive days. The 24-h dietary recall is a relatively quick and systematic evaluation method to acquire the recent information about the intake of food and is used widely in epidemiologic research(Reference Bross, Noori and Kovesdy11). The US Department of Agriculture developed the automated multiple pass method, which utilises a five-step, interviewer-administered, computer-based procedure for obtaining diet recalls. Details of this dietary assessment methodology have been published elsewhere(Reference Burrows, Martin and Collins12,Reference Conway, Ingwersen and Moshfegh13) . The calculation of nutrient intake was based on a dietary software, version 2.0 (Zhending). Nutrient models were sourced from the Chinese food consumption table developed in 2009 by the Center for Disease Control and Prevention of China. The dietary fibre intake was from foods (cereals, vegetables and fruits), not supplements. The intake of dietary fibre, protein and energy by the participants was normalised to the actual dry weight of the patient and presented in terms of g/kg per d and kJ/kg per d.
Study outcomes
All-cause mortality was defined as death from any cause, whereas CVD mortality was defined as death from myocardial infarction, heart failure, sudden cardiac death, stroke, cardiovascular haemorrhage and other vascular causes that are known.
Mortalities were confirmed either from hospital-provided death certificates or from a comprehensive consensus of the experts if death occurred out of hospital. All patients were followed up until death, transfer to kidney transplantation, peritoneal dialysis, end of the latest survey (July 2019) or censoring.
Statistical analysis
We assumed that the annual mortality rate among the participants with lower DFI was about 35 %, with a type I error rate of 0·025; enrolled 300 participants in lower DFI group and 600 participants in higher DFI group; would provide >80 % power to observe a hazard ratio (HR) of ≤0·75 for the comparison between lower v. higher DFI groups during a follow-up of about 3·5 years. Thus, given the possible rate of loss to follow-up, a sample size of about 1000 would be required.
Data are presented as means and standard deviations for continuous variables that are normally distributed, as medians and interquartile ranges for continuous variables that are skewed, and as frequencies and percentages for categorical variables. The difference among the tertiles of DFI levels was tested for categorical variables using the χ 2 test, and the Kruskal–Wallis test or ANOVA for continuous variables as suitable.
Variables that are known as traditional or suspected risk factors for the prognosis of haemodialysis patients(Reference Ma and Zhao14,Reference Pifer, Mccullough and Port15) or variables that showed significant differences among different DFI levels were chosen as covariates in this study. We first explored the association between DFI and mortality using thin-plate regression splines in generalised additive models implemented by the R package mgcv. Then univariate and multivariate Cox proportional hazard regression models (HR (OR) and 95 % CI) were used to evaluate the relation of DFI (tertiles and categories: tertile 1 v. tertiles 2–3) with all-cause and CVD mortality risk with and without adjustment for age, sex, dialysis centres, education level, smoking status, alcohol consumption, co-morbidities (diabetes, hypertension, history of CVD), BMI, waist:hip ratio, Kt:V ratio, dialysis duration, serum albumin, cholesterol, creatinine, CRP, dietary energy intake (DEI) and dietary protein intake (DPI). In Cox proportional hazards, time at risk was from the entry of the study until death, transferring to kidney transplantation, peritoneal dialysis or the end of the latest survey, whichever came first. The proportional hazards’ assumption was checked using statistical tests based on the scaled Schoenfeld residuals. Kaplan–Meier estimates were used to generate survival curves, and the log-rank test was applied to compare differences between DFI categories (tertile 1 v. tertiles 2–3) in survival. Furthermore, we added competing risk models to our analysis, treating non-CVD death as a competing event of CVD death. In competing risk models, cumulative incidence function was used to generate survival curves, and the differences among groups were recognised by Gray’s test using the R package cmprsk. The Fine and Gray proportional sub-distribution hazard model was performed to determine the 95 % CI and HR for CVD mortality associated with DFI using the R package mstate.
As additional exploratory analyses, possible modifications on the association between DFI (tertile 1 v. tertiles 2–3) and mortality risk were also evaluated by stratified analyses and interaction testing.
In all the analyses, a two-tailed P < 0·05 was regarded as statistically significant. All analyses were conducted using Empower (www.empowerstats.com) from X&Y Solutions Inc. and R software (http://www.R-project.org), version 3.5.3.
Results
Study participants and baseline characteristics
As illustrated in the flowchart (Fig. 1), a total of 1302 MHD patients participated in the survey, and 1044 patients were included in the final analysis. The baseline characteristics were similar among participants included and those not included in the current study (online Supplementary Table 1).

Fig. 1. Participant flowchart. CRP, C-reactive protein.
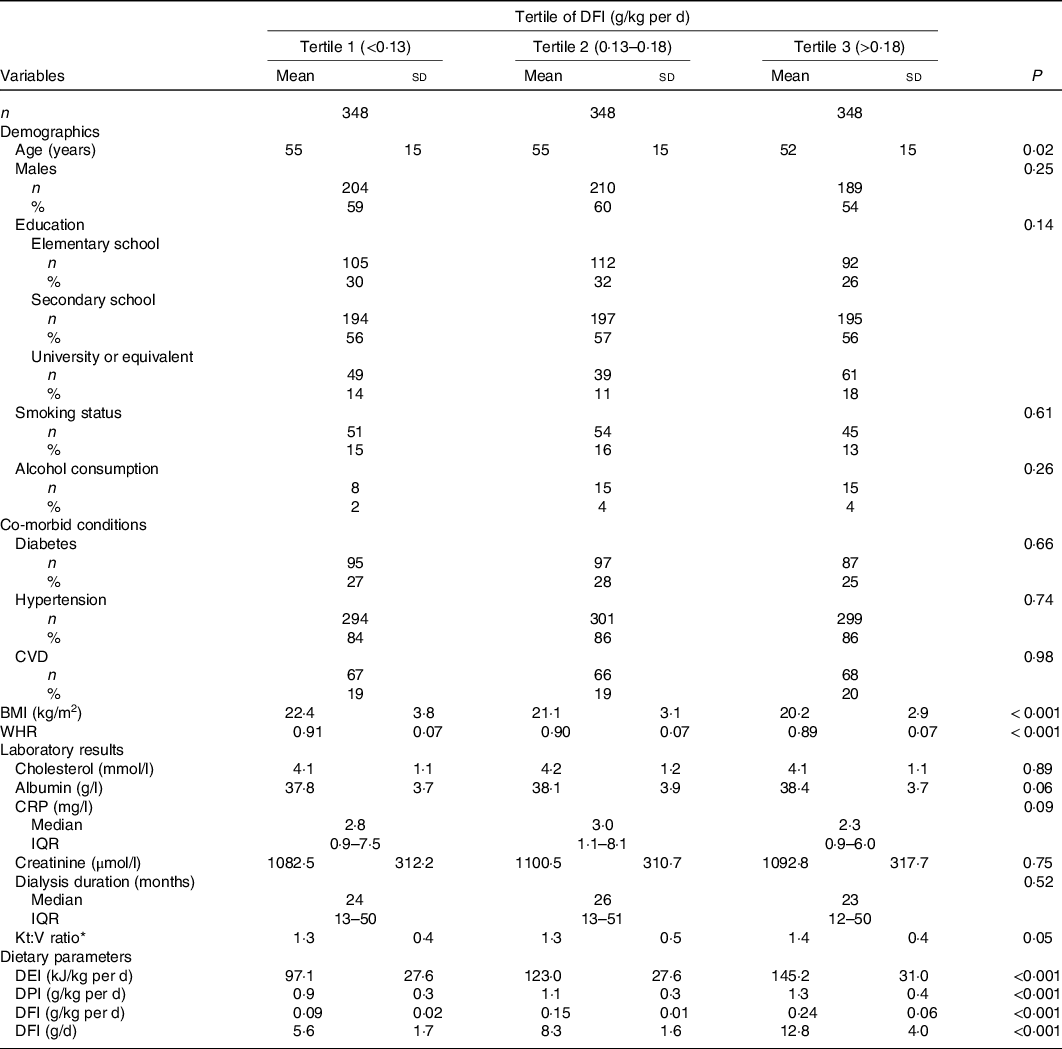
Supplementary Fig. 1 shows the distribution of DFI in the participants. Of the participants, 90 % consumed a value of DFI <14 g/d. The study population’s baseline characteristics according to the tertiles of DFI are shown in Table 1. In this study, the average age of the participants was 54 (SD 15) years, and 58 % of them were males. The mean DFI in the total population was 0·16 (SD 0·07) g/kg per d. Participants with higher DFI levels tended to be younger, had lower BMI and waist:hip ratio and had higher DEI, DPI and Kt:V ratio.
Table 1. Baseline characteristics of study participants according to dietary fibre intake (DFI) levels*
(Numbers and percentages; mean values and standard deviations; medians and interquartile ranges (IQR))

WHR, waist:hip ratio; CRP, C-reactive protein; DEI, dietary energy intake; DPI, dietary protein intake; BUN, serum blood urea nitrogen; UF, ultrafiltration.
* Kt:V ratio, Kt represents the effective urea clearance and duration of dialysis, and V represents the volume of distribution of urea in the body, calculated as Kt:V = –ln (post-BUN/pre-BUN – 0·008 × t) + (4–3·5 × post-BUN/pre-BUN) × UF /post-weight, where t is effective dialysis time.
Association between dietary fibre intake and the risk of outcomes
During a median follow-up duration of 46 months, all-cause mortality occurred in 354 (34 %) participants and 210 (59 %) deaths were due to CVD.
The association between DFI levels and CVD mortality appeared to be non-linear (online Supplementary Fig. 2). Accordingly, in the multivariable Cox regression analysis (Table 2), when DFI was assessed as tertile, the adjusted HR and 95 % CI of CVD mortality for participants in tertile 2 (0·13 to <0·18 g/kg per d) and tertile 3 (≥0·18 g/kg per d) were 0·72 (95 % CI 0·51, 1·02) and 0·68 (95 % CI 0·45, 1·02), respectively, compared with those in tertile 1. Consistently, a remarkably lower CVD mortality risk was found in tertiles 2–3 (≥0·13 g/kg per d; adjusted HR 0·71; 95 % CI 0·51, 0·97) compared with participants in tertile 1. Similar results were found in the competing risk analysis (online Supplementary Fig. 3 and Table 2). In the Cox proportional hazard regression models for the association between DFI (tertial 1 v. tertiles 2–3) and CVD mortality, no clear evidence was found against the proportional hazards’ assumption (P = 0·966).
Table 2. Univariate and multivariate Cox regression analyses of dietary fibre intake (DFI) in relation to mortality
(Hazard ratios (HR) and 95 % confidence intervals)

Ref, reference.
* Adjusted for dialysis centre, age, sex, education level, smoking status, alcohol consumption, dietary energy intake, dietary protein intake, BMI, waist:hip ratio, albumin, cholesterol, C-reactive protein (log-transformed), creatinine, dialysis duration, Kt:V ratio and history of hypertension, diabetes and CVD.
Kaplan–Meier curves of the cumulative event rate of CVD mortality for DFI categories (tertial 1 v. tertiles 2–3) are shown in Fig. 2. Consistently, the participants in tertiles 2–3 had a remarkably lower risk of mortality due to CVD (HR 0·74; 95 % CI 0·56, 0·98) compared with those in tertile 1.

Fig. 2. Kaplan–Meier estimates of all-cause mortality (a) and CVD mortality (b) according to dietary fibre intake categories. ![]() Tertile 1;
Tertile 1; ![]() tertile 2
tertile 2
A similar but non-significant trend was found for all-cause mortality (Fig. 2 and Table 2).
Stratified analysis of CVD mortality
Stratified analyses were conducted to assess the relationship between DFI and CVD mortality risk in various subgroups. As shown in Fig. 3, no variable significantly modified the correlation between DFI and CVD mortality for sex (male v. female), age (<60 v. ≥60 years), history of CVD (yes v. no), diabetes (yes v. no), BMI (<23 v. ≥23 kg/m2), CRP (<3 v. ≥3 mg/l)(Reference Pearson, Mensah and Alexander16), DPI (<1·2 v. ≥1·2 g/kg per d), DEI (<125·5 v. ≥125·5 kJ/kg per d), albumin (<35 v. ≥35 g/l) and dialysis duration (<24 v. ≥24 months) (all values of P for interaction >0·05). Further stratified analysis based on age, BMI, DPI and DEI tertiles also found no interaction effect (online Supplementary Fig. 4).

Fig. 3. Stratified analyses of the association between dietary fibre intake and the risk of CVD mortality. HR, hazard ratio; CRP, C-reactive protein; DPI, dietary protein intake; DEI, dietary energy intake.
Discussion
This multicentre prospective study was the first to show that high DFI correlated with a significantly decreased risk of CVD mortality after a follow-up of 46 months in MHD patients. Besides, the relation between DFI and CVD mortality was independent of sex, age, history of CVD, diabetes, BMI, CRP, DPI, DEI, albumin and dialysis duration.
Several previous reports have shown an inverse correlation between DFI and CVD mortality in general population. The NIH-AARP Diet and Health Study found that the multivariate relative risk for mortality due to CVD in the highest quintile were 0·76 (95 % CI 0·68, 0·85) for men and 0·66 (95 % CI 0·55, 0·79) for women compared to individuals in the lowest quintile of DFI(Reference Park, Subar and Hollenbeck17). A recent meta-analysis of twenty-two cohort studies examining the association of DFI with CVD mortality reported a 9 % reduction in mortality from CVD for each 7 g/d increase in DFI(Reference Threapleton, Greenwood and Evans8). The present study extends the existing evidence by demonstrating that the inverse association between DFI and CVD deaths is also existent in MHD patients. Consistently, a recent study in Hong Kong involving 127 peritoneal dialysis patients showed that an increase of dietary fibre by 1 g correlated with a reduced risk of major adverse cardiovascular events by 11 (95 % CI 0·81, 0·97) % after 18 months of follow-up(Reference Wang, Sea and Ng18).
The inverse association between DFI and CVD mortality in MHD could be explained by different mechanisms. A part of the protective effect of fibre intake in CVD mortality may be attributed to its regulation of gut microbiota. Fibres could decrease the generation of colon-derived uraemic toxins such as p-cresol sulphate and indoxyl sulphate by modifying microbiota populations of the gut(Reference Kieffer, Martin and Adams19). Through dialysis, it is difficult to get rid of these two uraemic toxins, which are related to CVD events in dialysis population(Reference Meijers, Bammens and De Moor20–Reference Poesen, Viaene and Verbeke22). Several small randomised trials involving haemodialysis patients had reported that supplementation with fibre correlates with reduced levels of indoxyl sulphate and p-cresol sulphate in the plasma(Reference Khosroshahi, Abedi and Ghojazadeh23–Reference Sirich, Plummer and Gardner25). In addition, the well-proven health effects of dietary fibre, such as inflammation status amelioration(Reference Krishnamurthy, Wei and Baird7,Reference Xie, Ge and Huang26) , blood lipid profile improvement(Reference Whitehead, Beck and Tosh27), blood pressure regulation(Reference Khan, Jovanovski and Ho28,Reference Sun, Shi and Wang29) and glycaemic control(Reference Weickert and Pfeiffer30), may contribute to reduced CVD mortality by slowing the progression of CVD.
Recommendations for fibre intake in the USA, Australia, New Zealand and most European countries are about 25–35 g/d for the general population(Reference Stephen, Champ and Cloran31). However, the recommendation for the CKD population is inconclusive(Reference Andrassy32–Reference Fouque, Vennegoor and ter Wee34). Only some guidelines recommend an exact value, such as that from the Italian Society of Nephrology, which recommends a DFI of 20–30 g/d based on a study that examined four different types of renal diets in the CKD population. Guideline from the National Health and Family Planning Commission of China recommends a DFI of 14 g/4184 kJ based on the recommended energy intake of CKD patients(Reference Cupisti, Brunori and Di Iorio35,36) . Current guidelines for dialysis patients lack specific recommendations for dietary fibre, and an optimal DFI value for dialysis patients still remains to be determined. It was notable that the mean fibre intake in our study was 8·9 g/d, which was relatively low compared with the recommendation from guidelines. Similarly, previous studies have found that patients receiving dialysis consume significantly lower amounts of dietary fibres compared with non-dialysis patients. These studies reported a daily fibre intake of 5–16 g/d in the haemodialysis population(Reference Therrien, Byham-Gray and Denmark37–Reference As’habi, Tabibi and Houshiar Rad40). Several factors are involved in the reduced intake of fibre in dialysis population. One reason may be the dietary restriction on vegetables and fruits, as patients receiving dialysis were required to decrease K and phosphate intake in order to prevent (or correct) hyperkalaemia and hyperphosphatemia. This may lead to a simultaneous reduction in dietary fibre(Reference Kalantar-Zade and Fouque41). Besides, the loss of appetite due to side effects during or after dialysis may also reduce fibre intake in these patients. Our current study further revelled the poor status of fibre intake and emphasised the significance of improving the intake of fibre in MHD patients. More studies are required to elucidate the suggested intake of fibre in dialysis and CKD populations.
Our study had several strengths that should be highlighted. First, this was the first multicentre cohort study conducted prospectively to explore whether the intake of dietary fibre correlated with CVD mortality in MHD patients and which had a comparatively long duration of follow-up with a large sample size. Second, detailed information, such as patients’ characteristics, co-morbidities and laboratory data, was collected. Dietary nutrients were also quantified by a validated 24-h dietary recall thrice rather than a single assessment. Finally, the association between DFI and mortality (CVD and all-cause) was examined and a comprehensive adjustment including of total energy and protein intake was made to minimise bias.
This study also had some limitations that should be addressed. First, as an observational study, residual confounding cannot be ruled out due to interference from factors that we did not determine, such as race/ethnicity, genetic factors and trimethylamine-N-oxide. However, our current study only included the Chinese and did not have detailed genetic information. Moreover, the relation between trimethylamine-N-oxide and mortality risk in MHD patients is inconclusive(Reference Zhang, Zou and Chen42,Reference Stubbs, Stedman and Liu43) and remains to be studied. Second, only patients with normal intake were included in this study; the results may not be applicable to patients receiving enteral or parenteral nutrition. The association between DFI and mortality in these patients needed to be further examined. Third, the assessment of each parameter was done at baseline, only once. More frequent measurements would have allowed a more accurate assessment of the DFI and mortality association. Fourth, our study could not distinguish dietary fibre into insoluble and soluble fibre and could not determine the respective food source. Different types and sources of fibre may have different effects on the prognosis of patients with MHD, which still needs to be verified. Finally, participants in our study had relatively lower fibre intake levels. Due to these limitations, confirmation of our findings through further studies is essential.
In conclusion, we first demonstrated that a higher intake of dietary fibre significantly correlated with a reduced risk of CVD mortality in MHD patients. These results provide new insights into the benefits of DFI in MHD patients. Future randomised clinical trials are needed to further explore this association.
Acknowledgements
We thank the participants, investigators, and staff for their contribution to this study.
This study was supported by the National Key Technology Support Program of China (grant number 2015BAI12B00), the High-Level Matching Funds of Nanfang Hospital (2 014 070), the Key Research Project of Southern Medical University (LC2019ZD005), the Major New Drug Creation Technology Major Project (2020zx09201017).
M. L. and Z. Z. L. designed research; Z. Z. L., Y. Y. Y., Y. H., J. Y. W., Y. M. L., S. L. Y., Y. X. L., Y. H. Z., Y. L., Z. H. L., Y. Z. K., Y. L., Q. W., F. N. L., S. H., A. Q. L., Q. J. W. conducted research; Z. Z. L. and X. H. Q. analysed data; Z. Z. L. wrote the paper; X. H. Q., F. F. H., Y. B. L. and M. L. revised the manuscript; M. L. had primary responsibility for final content. All authors read and approved the final manuscript.
X. Q. reports grants from the National Natural Science Foundation of China (81973133, 81730019). F. H. reports grants from the Science and Technology Planning Project of Guangzhou and the National Key Research and Development Program.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114521000210