Free radical damage is considered to cause cell and tissue damage and ultimately results in ageing and cell death(Reference Harman1). It has been shown that an overdose of d-galactose (DG) beyond the capacity of metabolism leads to the accumulation of galactitol, which in turn leads to osmotic stress and the generation of reactive oxygen species(Reference Wang2). In addition, DG is a reducing sugar that reacts with free amines of amino acids in proteins and peptides in vitro and in vivo to form advanced glycation end products(Reference Bucala and Cerami3). The subcutaneous (s.c.) administration of DG in C57BL/6J mice has been shown to enhance parameters of Alzheimer's disease(Reference Hsieh, Wu and Hu4) and to cause neurological impairment(Reference Cui, Zuo and Zhang5, Reference Zhang, Li and Cui6). However, systemic effects of this treatment on oxidative stress, especially in other mouse strains, have not been well established.
Fructo-oligosaccharides (FO), a prebiotic fibre with a well-recognised stimulatory effect on bifidobacteria(Reference Gibson7), has been incorporated into drinks and desserts to improve the bowel function and faecal microflora profile in older nursing-home adults(Reference Chen, Lu and Lin8, Reference Yen, Kuo and Tseng9). Besides its wide application in bowel irregularity, FO may modulate oxidative stress through its role in the gut. We have shown in vitro antioxidative capacity from bifidobacteria-fermented FO(Reference Wang, Lai and Chen10). A recent study has indicated that inulin, another oligofructose, ameliorated high-fat-induced oxidative damage and enhanced the gene expression of antioxidant enzymes not only in the colon, but also in the liver of Sprague–Dawley rats(Reference Wu and Chen11). A clinical study has indicated that FO supplementation in older nursing-home adults causes a reduction in plasma lipid peroxide, which is correlated with an increased faecal bifidobacteria population(Reference Yen, Kuo and Tseng9). All these studies have suggested that FO may ameliorate oxidative stress systemically.
Authors have recently demonstrated that s.c. DG treatment in BALB/cJ mice induced alterations in hepatic antioxidant enzyme activities, and dietary FO reversed these alterations(Reference Chen, Wang and Kuo12). However, the effects of DG on the formation of oxidative biomolecules in BALB/cJ mice have not been well studied, compared with older mice. Furthermore, the antioxidative effects of FO on these DG-induced alterations remain to be examined. There are two goals of the present study: (1) to compare the biomarkers of lipid, protein and DNA oxidation in tissues and plasma, and erythrocyte antioxidant enzymes between DG-treated young BALB/cJ mice and their natural ageing counterparts (aged control); (2) to determine the modulatory effects of FO and α-tocopherol (antioxidant control) on these DG-induced alterations.
Materials and methods
Animals and diets
Male BALB/cJ mice (BioLASCO, Taipei, Taiwan) were housed (two animals per cage) in solid-bottomed plastic cages with wood shavings for bedding in a room maintained on a 12 h light–12 h dark cycle (08.00–20.00 hours) at 24 ± 1°C and 50 % humidity at the Experimental Animal Center of Chung Shan Medical University. After a week of adaptation, mice (12 weeks of age, n 40) were randomly divided into four groups (n 10): vehicle+control (s.c. saline, basal diet); vehicle+DG (s.c. 1·2 g DG/kg body weight, basal diet); DG+FO (50 g active ingredients/kg basal diet); DG+vitamin E (2 g α-tocopherol/kg basal diet, as an antioxidant positive control). Then, the animals were killed after 52 d of treatment. The basal diet consisted of a ground rodent chow (Lab 5001; Purina Mills, St Louis, MO, USA) and sucrose that matched the digestible sugar present in the FO syrup (Institute of Microbial Resources, Taichung, Taiwan), as described previously(Reference Chen, Wang and Kuo12). FO (Institute of Microbial Resources) contains fifty-one (39 %, w/w, dry basis) oligomers including fructosylnystose, nystose and kestose; the remaining are made of simple sugars, as described previously(Reference Chen, Lu and Lin8). The mixed powder diet was then re-formed into a small dough with deionised water in order to balance the liquid content among the diets and to reduce spillage. The dose of FO and α-tocopherol used in the present study was shown to exert prebiotic(Reference Gibson7, Reference Yen, Kuo and Tseng9) and antioxidative(Reference Shih, Chang and Yang13) effects, respectively. Natural-ageing mice (NA group) that did not receive any injection were fed with a standard rodent diet (Lab 5001; Purina Mills) until the age of 39 weeks. They were then fed with the basal diet for 52 d and killed at the age of 47 weeks. All animals were allowed to have free access to water and food during the study. Animal care followed the guidelines of the National Research Council(14) and was approved by the Institutional Animal Care and Use Committee at the Chung Shan Medical University. Mice were anaesthetised with CO2 on day 52 after a 20 h fasting. After blood samples were collected from the right atrium into heparinised tubes, mice were transcardially perfused with ice-cold normal saline for 15 min. The cerebral cortex and hippocampus were dissected on an ice-cold plate, as described previously(Reference Wang, Wu and Liou15), and the liver was dissected into three portions. The brain and liver samples were frozen immediately and stored at − 80°C for further analyses of oxidative stress indices. The blood samples were mixed with an equal volume of Histopaque® (Sigma, St Louis, MO, USA) and centrifuged at 3000 g for 10 min at 4°C. The erythrocyte layers were washed with PBS, followed by the addition of a 4-fold volume of ice-cold distilled water, and then centrifuged at 10 000 g for 15 min at 4°C. The supernatant, i.e. erythrocyte lysate, was collected and stored at − 80°C for further analyses of antioxidant enzyme activities.
Malonaldehyde dimethyl acetal in the plasma, liver and cortex
Malonaldehyde dimethyl acetal (MDA) levels were determined using 1,1,3,3-tetraethoxypropane (Sigma) as the standard using the method described by Hong et al. (Reference Hong, Yen and Chang16). The hepatic tissues (0·2 g) were homogenised in 9-fold volumes of 0·1 m-potassium phosphate buffer containing 1·15 % (w/v) potassium chloride (pH 7·0) and centrifuged at 12 000 g for 10 min to obtain the supernatant. After incubation with 5-fold volumes of TCA (7·2 %, w/v, containing 1 %, w/v, potassium iodide) in ice for 10 min, the samples were centrifuged at 12 000 g for 10 min. An aliquot (0·5 ml) of this supernatant was mixed with 1 ml of 0·6 % (w/v) thiobarbituric acid (TBA) at 95°C for 1 h, and the MDA–TBA adduct was extracted with n-butanol. The MDA–TBA adduct was eluted with a mixture of phosphate buffer (50 mm) and methanol (65:35, v/v) at 0·8 ml/min in a HPLC system (Jasco, Tokyo, Japan) equipped with a C18 reverse-phase column (LiChroCART 250-4; Merck, Darmstadt, Germany) capped with a guard column (LiChrospher 100 RP-18e; Merck). The quantification of the MDA–TBA adduct was determined at 532 nm and quantified with a standard curve. All samples were determined in triplicate assays.
Protein carbonyls in the liver and hippocampus
Carbonyl proteins, a protein oxidation index, were determined according to the method described by Reznick & Packer(Reference Reznick and Packer17). The liver tissue (0·2 g) and hippocampus were homogenised (20 mm-Tris buffer, 0·137 m-NaCl, 10 % glycerol, 1 % Triton X-100, 2 mm-EDTA, 1 % protease, pH 8·0) and centrifuged at 10 000 g for 15 min at 4°C. The supernatant was treated with 1 % (w/w) streptomycin sulphate for 15 min to remove nucleic acids. The carbonyl groups derivatised to 2,4-dinitrophenylhydrazone by the reaction with 2,4-dinitrophenylhydrazine were determined at 380 nm. Protein contents were determined with the Bradford method using a protein reagent (Bio-Rad Laboratories, Herculus, CA, USA), with bovine serum albumin as the standard. All samples were determined in triplicate assays.
Hepatic mitochondrial 8-oxo-deoxyguanosine
Hepatic mitochondria were isolated according to the method described previously(Reference Lai and Clark18). Briefly, liver tissue (~0·1 g) was homogenised in a 4-fold volume of isolation buffer (0·25 m-sucrose, 10 mm-Tris and 0·5 mm-EDTA, pH 7·4). After the nuclei and cell debris were sedimented by centrifugation at 2000 g for 3 min at 4°C, the supernatant was subjected to a further centrifugation at 12 500 g for 8 min. The crude mitochondrial DNA was isolated with REzol™ C&T reagent (Protech Systems Co., Taipei, Taiwan) following the manufacturer's protocol. The isolated DNA (200 μg) was treated with 20 U nuclease P1 and 1·5 IU alkaline phosphatase(Reference Shigenaga, Park and Cundy19), and was analysed using a competitive enzyme immunoassay (Cayman Chemical Co, Ann Arbor, MI, USA) according to the protocol provided by the manufacturer. All samples were determined in duplicate assays.
Erythrocyte antioxidant enzyme activities
Superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase activities were determined in erythrocyte lysates. SOD activity was measured based on the competition for superoxide radicals between SOD and a tetrazolium salt(Reference Flohé, Becker, Brigelius, Miquel, Quintanilha and Weber20). GPx activity was measured indirectly by a coupled reaction with glutathione reductase that converted the oxidised glutathione to its reduced form with a concomitant oxidation of NADPH to NADP+ (Reference Flohé and Gunzler21). Catalase activity was measured based on the transformation from methanol to formaldehyde that reacted with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole(Reference Johansson and Håkan Borg22). Hb contents of the erythrocyte lysate were analysed following the manufacturer's protocol (Sigma). Enzyme activity was expressed as IU/mg Hb. All samples were determined in triplicate assays.
Statistical analysis
Data are expressed as means with their standard errors, and analysed using SPSS version 12 (SPSS, Inc., Chicago, IL, USA). Differences between groups were analysed by one-way ANOVA, followed by the least significant difference test. Differences were considered significant at P < 0·05.
Results
Weight gain, feed intake and feed efficiency
Data of the growth and feed intake in young mice receiving 52 d of s.c. vehicle or DG injection are shown in Table 1. Body-weight gain was the lowest in DG-treated mice without any supplementation (DG+control group), and the greatest in the DG+FO group. Daily feed intake was the lowest in the DG+control group and similar among the other groups. Feed efficiency (body-weight gain/feed intake) was the greatest in the DG+FO group and was similar among the other groups. Age and diet for the NA group were different from the other treated groups. Therefore, the related data are not shown in Table 1. The body weight of NA mice was 34·6 (se 0·3) g at the end of the experiment; their daily food intake was 4·0 (se 0·2) g during the last week of the experiment.
Table 1 Body-weight gain, feed intake and feed efficiency in various groups of BALB/cJ mice
(Mean values with their standard errors, n 10)
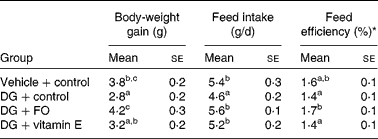
DG, d-galactose; FO, fructo-oligosaccharide.
a,b,c Mean values with unlike superscript letters within a column were significantly different (P < 0·05; ANOVA followed by the least significant difference test).
* Feed efficiency (%) = body-weight gain (g/d)/feed intake (g/d) × 100 %.
Plasma, liver and cerebral cortex malonaldehyde dimethyl acetal
The DG treatment alone significantly elevated the formation of MDA in the plasma, liver and cerebral cortex when compared with the vehicle-treated counterpart (Table 2). The formation of MDA in the plasma, liver and cerebral cortex was similar to what was observed in the NA group. Both FO and α-tocopherol attenuated the effect of DG on the plasma MDA level, and completely diminished its effect in the liver and cerebral cortex. α-Tocopherol further suppressed the cortex MDA level to only 83·1 % of that in the vehicle+control group.
Table 2 Malonaldehyde dimethyl acetal levels in the plasma, liver and cerebral cortex in various groups of BALB/cJ mice
(Mean values with their standard errors, n 10)
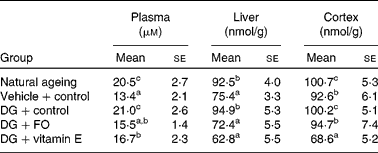
DG, d-galactose; FO, fructo-oligosaccharide.
a,b,c Mean values with unlike superscript letters within a column were significantly different (P < 0·05; ANOVA followed by the least significant difference test).
Protein carbonyls in the liver and hippocampus
Both hepatic and hippocampus protein carbonyl levels were similar between the NA and DG+control groups (Table 3). Both FO and α-tocopherol diminished the effect of the DG treatment on hepatic protein carbonyl levels, and further reduced the levels to about 80·0 and about 56·0 % of that shown in the vehicle+control group, respectively. In the hippocampus, both FO and α-tocopherol diminished the effect of the DG treatment on protein carbonyl levels, while α-tocopherol further suppressed the levels lower than that in the vehicle+control group.
Table 3 Protein carbonyl levels in the liver and hippocampus in various groups of BALB/cJ mice
(Mean values with their standard errors, n 10)
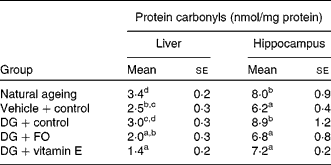
DG, d-galactose; FO, fructo-oligosaccharide.
a,b,c,d Mean values with unlike superscript letters within a column were significantly different (P < 0·05; ANOVA followed by the least significant difference test).
Hepatic mitochondrial 8-oxo-deoxyguanosine
The oxidative modification of hepatic mitochondrial DNA is shown in Fig. 1. The mitochondrial 8-oxo-deoxyguanosine (8-oxo-dG) levels in the NA and DG+control groups were significantly elevated by about 80 and 40 %, respectively, compared with that in the vehicle+control group. FO and vitamin E did not just diminish the effect of DG, but further suppressed the formation of 8-oxo-dG to levels lower than that shown in the vehicle+control group.

Fig. 1 Hepatic mitochondrial 8-oxo-deoxyguanosine (8-oxo-dG) levels in natural ageing mice (47 weeks old; NA group) or young male BALB/cJ mice (about 19 weeks old) subcutaneously treated with vehicle (saline) or d-galactose (DG). The vehicle-treated group was fed the control diet (vehicle+control group) while DG-treated mice were fed with the control diet (DG+control group), the fructo-oligosaccharide (FO) diet (50 g active ingredients/kg diet; DG+FO group) or the vitamin (Vit) E diet (2 g α-tocopherol/kg diet, as an antioxidant positive control; DG+Vit E group). Values are means, with their standard errors represented by vertical bars (n 10). a,b,c,d,e Mean values with unlike letters were significantly different (P < 0·05).
Erythrocyte antioxidant enzyme activities
SOD activities in both NA and DG+control groups were significantly greater than that in the vehicle+control group (Table 4). Dietary FO and vitamin E normalised the alteration induced by the DG treatment. GPx activity was drastically elevated by the DG treatment to more than 3-fold of that in the vehicle+control group, but only slightly greater in the NA group. α-Tocopherol, but not FO, significantly reduced the altered GPx activity induced by the DG treatment. Catalase activity was the lowest in the NA group, and the DG treatment did not modulate this enzyme activity when compared with the vehicle treatment. Dietary FO and α-tocopherol significantly increased the catalase activity by 20·7 and about 50·0 %, respectively, compared with that in the DG+control group.
Table 4 Erythrocyte antioxidant enzyme activities in various groups of BALB/cJ mice
(Mean values with their standard errors, n 10)
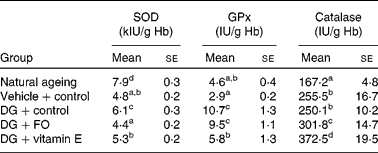
SOD, superoxide dismutase; GPx, glutathione peroxidase; DG, d-galactose; FO, fructo-oligosaccharide.
a,b,c,d Mean values with unlike superscript letters within a column were significantly different (P < 0·05; ANOVA followed by the least significant difference test).
Discussion
DG treatment in C57BL/6J mice has been shown to enhance oxidative stress and neurological dysfunction(Reference Hsieh, Wu and Hu4–Reference Zhang, Li and Cui6). The physiological responses to DG-induced oxidative stress have not been well established in various strains of mice. Therefore, in the present study, we thoroughly determined the status of lipid, protein and DNA oxidation, and erythrocyte antioxidant enzymes in older mice (NA group, 47 weeks old), younger mice (vehicle+control group, about 19 weeks old) and DG-treated young BALB/cJ mice. The results indicated that all of the oxidative products measured in the present study increased with age. DG treatment increased the levels of MDA in the plasma, liver and brain, and the levels of hippocampus protein carbonyls and hepatic mitochondrial 8-oxo dG to the same extent as observed in older mice. Therefore, the present study suggests that DG treatment accelerates ageing by increasing systemic oxidative stress, and DG-treated BALB/cJ mice could be another experimental model of oxidative-induced ageing.
The older population may be confronted with limited intake of fibre-rich food and associated antioxidants(Reference Ervin and Dye23, Reference Hildebrandt, Dominguez and Schork24). Insufficient intake of dietary fibre jeopardises bowel function while decreased antioxidant intake hampers the defence machinery against free radicals. Fructo-oligosaccharide is considered to be a functional fibre that normalises bowel function and stimulates the growth of bifidobacteria in the constipated older population(Reference Chen, Lu and Lin8, Reference Yen, Kuo and Tseng9). We have preliminarily found that FO (5 %, w/w diet), similar to the antioxidant α-tocopherol (0·2 %, w/w diet), normalised DG-induced alterations of hepatic SOD and GPx activities in BALB/cJ mice(Reference Chen, Wang and Kuo12). In agreement with that, the present results indicate that FO, generally similar to α-tocopherol, successfully diminishes the DG-induced oxidative modifications of lipids in the plasma, liver and cortex, proteins in the liver and hippocampus, and hepatic mitochondrial DNA, and normalises the DG-induced alteration of erythrocyte SOD activities. Since FO is a well-recognised prebiotic fibre, the present study suggests that FO modulates systemic oxidative stress via its role in the intestine.
The central nervous system is vulnerable to oxidative insult on account of the high rate of oxygen utilisation, the relatively poor concentrations of classical antioxidants, antioxidant enzymes and high phospholipid content(Reference Sayre, Perry and Smith25). Increased oxidative damage is associated with age-related neurodegenerative diseases, such as Alzheimer's disease(Reference Sayre, Perry and Smith25). In the present study, the DG treatment enhanced the levels of cerebral cortex MDA and hippocampus protein carbonyls, suggesting that this treatment caused oxidative damages in the brain and could result in neurological alteration. Furthermore, this is the first study indicating that dietary supplementation of FO could protect the brain from oxidative damage. In the present study, α-tocopherol supplementation exerted strong protective effects on oxidative modifications of lipids and proteins in the brain, suggesting its role in preventing oxidative stress-related neurological alteration. These results supported the role of vitamin E in protecting human cognitive function(Reference Morris, Evans and Bienias26) and preventing Alzheimer's disease(Reference Morris, Evans and Tangney27). Although FO suppressed oxidative damage in the brain, its effect on neurological dysfunction and the mechanism underlying these effects remains to be investigated.
The liver is a major organ involved in carbohydrate, lipid and protein metabolism that produces abundant free radicals. It has been reported that age and DG treatment decrease major antioxidant enzymes in the liver(Reference Chen, Wang and Kuo12). In the present study, we specifically determined the effects of DG treatment on hepatic mitochondrial DNA damage, in addition to MDA and protein carbonyl levels, and further examined whether FO and α-tocopherol could suppress the DG-induced alterations. We found that hepatic levels of mitochondrial 8-oxo-dG and MDA were significantly elevated in both NA and DG+control groups, and the DG treatment slightly enhanced the hepatic protein carbonyl levels, which is in agreement with lowered hepatic SOD activities as shown in our previous study(Reference Chen, Wang and Kuo12). FO and α-tocopherol reversed the DG-induced elevations in the mitochondrial 8-oxo-dG, MDA and protein carbonyl levels in the present study, which are in agreement with their effects on normalising the DG-induced reduction in hepatic SOD levels(Reference Chen, Wang and Kuo12). Therefore, the present study confirmed a FO- and α-tocopherol-reduced hepatic oxidative stress induced by DG treatment in BALB/cJ mice via elevating the hepatic antioxidant enzyme activities.
The present study indicates that plasma lipid peroxide is enhanced by age and DG treatment, similar to what was observed previously in C57BL/6J mice(Reference Hsieh, Wu and Hu4). FO supplementation reduced the MDA level, in agreement with its effect in nursing-home residents(Reference Yen, Kuo and Tseng9). Erythrocyte antioxidant defence systems are confronted with oxidative challenges from plasma free radicals, macrophages, auto-oxidation of Hb, and potential peroxidation of membrane lipids(Reference Halliwell and Gutteridge28). However, the effects of age and DG treatment on blood erythrocyte antioxidant enzyme activities have not been well studied. A previous study has examined age-related changes in erythrocyte antioxidant enzyme activities in Sprague–Dawley male rats and found that erythrocyte SOD activity increased, while GPx and catalase activities decreased, with age(Reference Rho and Kim29). The present results indicate similar age-dependent changes in erythrocyte SOD and catalase activities, but GPx did not decrease with age. These differences between studies may result from differences in animals, diet and age. In the present study, the DG treatment increased erythrocyte SOD activity, which was diminished by FO and α-tocopherol to the level observed in the vehicle+control group. The DG-induced augmentation of erythrocyte GPx was suppressed only in mice supplemented with α-tocopherol, but not with FO, which could be due to the strong antioxidant effect of α-tocopherol on the peroxidation of erythrocyte membrane lipids. On the other hand, the DG treatment did not reduce erythrocyte catalase activity when compared with the control, but FO and α-tocopherol further elevated the activity. Antioxidants such as β-carotene and canthaxanthin have been shown to increase erythrocyte catalase activities(Reference Shih, Chang and Yang13), which suggest that FO enhanced the erythrocyte antioxidant defence system in a manner similar to antioxidants.
The present study further indicates the preventive and systemic effects of FO on DG-induced oxidative modifications of lipids, proteins and DNA. Although mechanisms for the in vivo antioxidative ability of FO remain unclear, it is likely to be related to its prebiotic effect and its fermentation products. Several studies have indicated in vitro antioxidative capacities of lactic acid bacteria(Reference Lin and Yen30, Reference Zanoni, Pompei and Cordisco31). We have also reported that the effect of FO on reducing plasma lipid peroxide was associated with increases in the faecal bifidobacteria population(Reference Yen, Kuo and Tseng9). In addition, previous studies have indicated that fermentation of FO by several species of bifidobacteria eliminated free radicals(Reference Wang, Lai and Chen10). Therefore, we suggest that FO may diminish DG-induced oxidative stress by contributing antioxidants produced from its fermentation in the colon, and by stimulating the proliferation of colonic bacteria that exert antioxidative capacity.
In conclusion, the results of the present study indicate that s.c. administration of DG in BALB/cJ mice generally enhances parameters of oxidative stress in the plasma, liver and brain, to levels similar to that observed in older mice. FO, similar to the antioxidant α-tocopherol, was found to effectively reduce the systemic oxidative stress induced by the DG treatment, which is probably due to its fermentation in the colon and the proliferation of colonic bacteria that exert antioxidative capacity.
Acknowledgements
The present study was supported by grant 98-CCH-CSMU-01 co-funded by the Changhua Christian Hospital and Chung Shan Medical University. C.-H. H. conducted the study. H.-L. C. designed and carried out the study, and wrote the manuscript. C.-H. W. collated and analysed the data, and co-wrote the manuscript. Y.-W. K. carried out the study. Y.-J. H. assisted in the mouse brain dissection and blood collection. The sponsor of FO by the Institute of Microbial Resources was greatly appreciated. All authors read and approved the findings of the study. There are no conflicts of interest.









