Poultry coccidiosis, caused by protozoa of the genus Eimeria, is one of the most frequently reported parasitic diseases and has substantial economic impact on the poultry industry( Reference Lillehoj, Min and Dalloul 1 – Reference Shivaramaiah, Barta and Hernandez-Velasco 3 ). Eimeria parasites invade the intestinal mucosa and induce villus damage and crypt dilation( Reference Fernando and McCraw 4 – Reference Tan, Applegate and Liu 6 ), resulting in poor nutrient digestion and absorption, and thus growth depression( Reference Tan, Applegate and Liu 6 , Reference Adedokun, Ajuwon and Romero 7 ). In order to defend against the parasite and acquire protective immunity, the host elicits a series of non-specific and specific immune responses( Reference Lillehoj, Min and Dalloul 1 – Reference Shivaramaiah, Barta and Hernandez-Velasco 3 , Reference Collier, Hofacre and Payne 8 ). The non-specific responses include increasing mucus production by goblet cells (GC)( Reference Collier, Hofacre and Payne 8 ) and activating phagocytosis by macrophages and dendritic cells( Reference Dalloul and Lillehoj 2 , Reference Shivaramaiah, Barta and Hernandez-Velasco 3 ), whereas the specific responses are primarily conferred by T lymphocytes and, to a lesser extent, by antibodies from B lymphocytes( Reference Lillehoj, Min and Dalloul 1 – Reference Shivaramaiah, Barta and Hernandez-Velasco 3 ). Therefore, dietary factors contributing to intestinal immune competence may play a role in protecting against coccidiosis.
Threonine (Thr) is one nutrient that particularly contributes to gut barrier integrity and its associated immune system. The most studied proteins related to intestinal Thr utilisation are the mucins, where Thr constitutes 28–35 % of the total amino acids( Reference Sandberg, Emmans and Kyriazakis 9 , Reference Nichols and Bertolo 10 ). The reduction in mucin synthesis under dietary Thr restriction has been demonstrated in rats( Reference Faure, Moennoz and Montigon 11 ), pigs( Reference Law, Bertolo and Adjiri-Awere 12 ), chickens( Reference Horn, Donkin and Applegate 13 ) and ducks( Reference Horn, Donkin and Applegate 13 , Reference Zhang, Xu and Doster 14 ). As the major constituent of mucus layers, decreased mucin production may account for the impairment of gut barrier function. Hamard et al. ( Reference Hamard, Mazuraisc and Boudry 15 ) showed that moderate Thr deficiency increased ileal paracellular permeability of piglets, along with gene expression changes of tight junction (TJ) proteins. In addition to mucins, Thr is an important structural component of Ig, accounting for 7–11 % of the total amino acids( Reference Sandberg, Emmans and Kyriazakis 9 ). Moreover, Thr may contribute to lymphocyte proliferation and activation. In vitro, Thr was demonstrated to be consumed by lymphocytes to maintain their proliferation and antibody production( Reference Duval, Demangel and Munier-Jolain 16 ). In addition, Thr starvation in culture medium depressed lymphocyte proliferation in response to mitogens( Reference Taudou, Wiart and Panijel 17 ). In animal studies, Thr is known to affect the production and activity of intestinal IgA in laying hens( Reference Azzam, Zou and Dong 18 ) and serum IgY in ducks( Reference Zhang, Xu and Doster 14 ). Overall, Thr is critical to maintain gut barrier function and its underlying immune competence.
The magnitude of coccidial pathogenesis depends largely on the successful invasion of parasites inside the host( Reference Shivaramaiah, Barta and Hernandez-Velasco 3 ). As the initial parasite–host interface, the mucus layer has the function to prevent Eimeria oocyst invasion into intestinal epithelial cells( Reference Tierney, Matthews and Carrington 19 ). The decrease of this mucus layer may lead to an increased susceptibility of birds to coccidiosis infection. Such a decrease can be observed after 24 h of feed withdrawal in birds( Reference Thompson and Applegate 20 ). In addition, this reduction in mucus content may worsen Thr deficiency under coccidia-induced mucogenesis( Reference Collier, Hofacre and Payne 8 ). Given the critical role that Thr plays in gut barrier and immune functions, it was hypothesised that Thr deficiency would exacerbate the intestinal damage induced by combined feed withdrawal and coccidial infection. Therefore, in this study, feed was withdrawn for 24 h as a predisposing factor to induce the loss of gut barrier integrity, and then coccidial vaccines were gavaged to establish a model of coccidiosis to determine the effect of Thr deficiency on intestinal morphology, barrier function, lymphocyte profile and cytokine gene expression of broiler chicks.
Methods
Birds, diets and experimental design
Animal care and use procedures were reviewed and approved by the Purdue University Animal Care and Use Committee. A total of 224 1-d-old male broiler chicks (Ross 708, hatching body weight (BW) was 33·8 (sem 0·2) g) were obtained from a commercial hatchery, weighed and allocated into groups to ensure similar initial weight in each group. All chicks were housed in electrically heated battery cages (Alternative Design Manufacturing and Supply Inc.) until 21 d of age. The battery cage temperature was maintained at 35±1°C for the 1st week and was gradually decreased to 27°C in the 3rd week. The lighting schedule was 22 h light–2 h dark throughout the experiment. Feed and water were provided ad libitum. Chicks were inspected daily for any health problems, and mortality was recorded as it occurred.
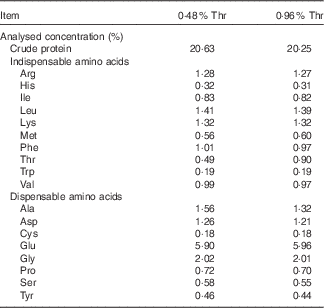
The completely randomised design consisted of a 2×2 factorial arrangement of treatments to evaluate dietary Thr concentrations (4·8 or 9·6 g/kg) with or without combined challenge (24-h feed withdrawal and coccidial vaccine challenge). Overall, there were four treatment groups with eight replicate cages per treatment and seven birds per cage. In order to obtain the low-Thr diet, the crude protein was formulated at a concentration of 210 g/kg. The other nutrients were formulated to meet or exceed the requirements of broilers according to the National Research Council( 21 ). A basal diet was initially mixed, and final diets were obtained by adding 98·0 % of the basal mix to a premix (2·0 %) containing different concentrations of Thr, Ala and maize starch, where Ala was added to make all diets isonitrogenous, and maize starch served as a filler. The ingredient composition used in this study is shown in Table 1. Dietary N (method 990.03; AOAC International, 2006) and amino acid (method 982.30 E(a,b,c); AOAC International, 2006) were analysed at the University of Missouri Experiment Station Chemical Laboratories, Columbia. The analysed dietary amino acid concentrations are presented in Table 2. Dietary Thr was analysed to be 4·9 or 9·0 g/kg, which is 61·25 or 112·5 % of the Thr requirement for broilers( 21 ), respectively.
Table 1 Feed ingredients and chemical composition of diets on an as-fed basis
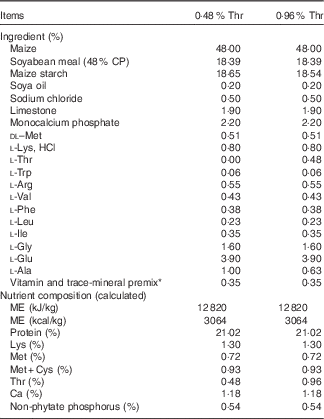
CP, crude protein; ME, metabolisable energy.
* Supplied the following per kg of diet: vitamin A, 13 233 IU; vitamin D3, 6636 IU; vitamin E, 44·1 IU; vitamin K, 4·5 mg; thiamine, 2·21 mg; riboflavin, 6·6 mg; pantothenic acid, 24·3 mg; niacin, 88·2 mg; pyridoxine, 3·31 mg; folic acid, 1·10 mg; biotin, 0·33 mg; vitamin B12, 24·8 µg; choline, 669·8 mg; Fe from ferrous sulphate, 50·1 mg; Cu from copper sulphate, 7·7 mg; Mn from manganese oxide, 125·1 mg; Zn from zinc oxide, 125·1 mg; I from ethylenediamine dihydroidide, 2·10 mg; Se from sodium selenite, 0·25 mg.
Table 2 Analysed amino acid concentrations of diets on an as-fed basis
Feed withdrawal, coccidial infection and sampling
At 13 d of age, birds in the challenged groups were feed deprived for 24 h and then gavaged with a coccidial vaccine at 25× dose (Coccivac®-B; Merck Animal Health), which contained live oocysts of Eimeria acervulina, Eimeria mivati, Eimeria maxima and Eimeria tenella. In our previous studies, 20×( Reference Tan, Applegate and Liu 6 ) and 25× (Grenier et al., unpublished results) doses of Coccivac®-B induced moderate intestinal damage and immune response. Birds in the control groups were not subjected to feed withdrawal, and were orally gavaged with water at 14 d of age. Cage weight and feed intake (FI) were measured at 0, 13 and 21 d of age for BW gain, FI and feed:gain. Average FI and BW gain were corrected for mortality. Excreta were collected for 1 d at 21 d of age, and stored at 4°C for oocyst counting. In addition, 1 bird/cage was randomly selected and euthanised with CO2 for tissue sampling at 14 d of age (after the 24-h feed withdrawal and before the coccidial vaccine challenge for the challenged groups) and at 21 d of age (7 d after coccidial vaccine challenge for the challenged groups). At each age, the same bird was used for the different analyses. At 14 and 21 d of age, gut permeability and concentrations of ileal mucosal barrier proteins were determined. In addition, at 21 d, intestinal gross lesion, jejunal morphology, lymphocyte profile and cytokine gene expression in caecal tonsil (CT) were determined.
Oocyst counting and lesion score
Oocyst counting was performed as described by Hodgson( Reference Hodgson 22 ) with modifications (Grenier et al., unpublished results). In brief, excreta within each cage were pooled together and mixed. Excreta/cage (approximately 500 mg) were weighed, passed and stirred through a sieve with 300 ml saturated NaCl solution. A sample of the mixture was transferred into the two chambers of a McMaster slide (FEC source). After 5 min, the four species of Eimeria oocysts were counted under a microscope at 10× magnification. The number of oocysts was expressed as oocyst per gram of faeces. In addition, gross lesions (1 bird/cage at 21 d of age) were scored in the duodenum, mid-jejunum and caecum according to Conway & McKenzie( Reference Conway and McKenzie 23 ), as the four species present in the live vaccine target the duodenal loop and upper jejunum (E. acervulina and E. mivati), mid-intestinal area (E. maxima) and the caecum (E. tenella).
Gut permeability
Fluorescein isothiocyanate-dextran (FITC-d) was administered orally, and its appearance in blood was used as a marker for TJ permeability as described previously( Reference Vicuña, Kuttappan and Tellez 24 ). At 14 and 21 d of age, FITC-d (2·2 mg/bird, molecular weight 3000–5000; Sigma Aldrich) was orally gavaged to 1 bird/cage, 2 h before birds were euthanised. Blood was collected via the jugular vein after euthanasia, kept at room temperature for 3 h to allow clotting and then centrifuged (1000 g for 15 min) to separate serum. Fluorescence levels of diluted serum (1:1 in PBS) were measured at an excitation wavelength of 485 nm and emission wavelength of 528 nm (Synergy HT, Multi-mode microplate reader; BioTek Instruments), and FITC-d concentration per ml of serum was calculated based on a standard curve.
Mucosal sample analysis
Mucosa samples from 1 bird/cage were scraped from 10 cm of the ileum (5 cm proximal to the Meckel’s diverticulum) at 14 and 21 d of age. Mucin from ileal mucosa was extracted by homogenisation in 0·2 m-NaCl containing protease inhibitors and 0·02 % NaN3 for 30 s, and subsequent centrifugation at 6000 g for 30 min( Reference Mantle and Allen 25 ). The supernatant was collected to determine soluble mucin concentrations by Alcian blue( Reference Deters, Petereit and Schmidgall 26 ) with modifications. In brief, 200 μl samples of homogenised supernatant were incubated with 50 µl Alcian blue reagent (100 mg Alcian blue in 100 ml 0·2 m-sodium acetate, 50 mm MgCl2, pH 2·5) with shaking for 2 h at room temperature. The mixture was centrifuged (10 min at 12 500 g ), and the absorbance of 200 μl of the clear supernatant (1:1 diluted) was measured at 595 nm. The residual Alcian blue in the supernatant correlated inversely with the amount of precipitated mucin. Calibration curves were constructed using commercial porcine mucin (Sigma Aldrich). Production of mucosal secretory IgA and IgM (Bethyl Laboratories), polymeric Ig receptor/secretory component (pIgR/SC; MyBioSource) and claudin-1 (MyBioSource) was determined by ELISA following the manufacturer’s protocol. The total protein in the mucosal homogenates was measured colorimetrically based on the Bradford dye-binding method using a commercially available kit (Bio-Rad) with bovine serum albumin as the standard.
Intestinal morphological analysis
The mid-jejunum from 1 bird/cage was collected at 21 d of age. Tissue samples were fixed in 10 % buffered formalin and were paraffin embedded. One section/bird (5-μm thick) was stained with Alcian Blue/PAS (periodic acid-Schiff) Stain Kit (Newcomer Supply) following the manufacturer’s protocol. After staining, treatment-blind histological analysis was performed on six complete and well-oriented villi and six crypts per section. Sections were assessed for villus height (VH), crypt depth (CD), GC counts (per villus) and GC density (per µm of VH) by light microscopy.
Lymphocyte preparation and flow cytometric analysis
At 21 d of age, the left CT from 1 bird/cage was cut and placed in tubes of ice-cold Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco) for immediate cell collection. Single-cell suspensions of CT were carried out as described in our previous study( Reference Xu, Eicher and Applegate 27 ) with modifications. In brief, CT samples were gently pressed over a 40-μm cell strainer (Fisher Scientific) into a petridish with RPMI 1640 medium. Cells were collected, washed twice and re-suspended in RPMI 1640 medium at a final concentration of 107 cells/ml. Next, 500 μl of cell suspensions was aliquoted and stained as follows: (1) control to determine background fluorescence; (2) anti-chicken CD4 labelled with phycoerythrin (PE, 0·2 μg antibody/106 cells) and anti-chicken CD8 labelled with FITC (1 μg antibody/106 cells); and (3) anti-chicken CD3 labelled with FITC (1 μg antibody/106 cells) and anti-chicken Bu-1 labelled with PE (0·2 μg antibody/106 cells). All antibody reagents were purchased from Southern Biotech. After 1 h of incubation with antibodies, the cells were washed twice with Hanks’ buffered salt solution (HBSS; Gibco). At the completion of the staining and washing procedure, the cells were re-suspended in 500 µl of HBSS with 2 % paraformaldehyde, held at 4°C and protected from light for analysis the next day using flow cytometry (C6 BD Accuri Cytometer, Inc.). Data were collected on 10 000 cells per sample. Lymphocyte gate was set according to the forward scatter and side scatter properties of chicken leucocytes.
Total RNA extraction and RT
At 21 d of age, the mid-jejunum and the right CT from 1 bird/cage were placed in RNALater (Ambion) overnight at 4°C and frozen at −80°C until RNA extraction. Total RNA was extracted from the tissues using TRIzol reagent (Invitrogen) following the manufacturer’s protocol. RNA concentrations were determined by NanoDrop 1000 (Thermo Scientific) and RNA integrity was verified by 1 % agarose gel electrophoresis. To eliminate DNA contamination, extracted RNA was purified with DNA-free DNase Treatment and Removal Kit (Ambion). Next, 2 mg of total RNA from each sample was reverse transcribed into complementary DNA (cDNA) using the M-MLV reverse transcription system (Promega), and cDNA was then diluted 1:10 with nuclease-free water (Ambion) and stored at −20°C until use.
Quantitative real-time PCR analysis
Real-time PCR was performed on the Bio-Rad iCycler with the Faststart SYBR green-based mix (Applied Biosystems). PCR programmes for all genes were designed as follows: 10 min at 95°C, forty cycles at 95°C for 30 s, primer-specific annealing temperature for 30 s and 72°C for 30 s, followed by melting curve analysis. The primer sequences used in this study are listed in Table 3. Primer specificity and efficiency (90–110 %) were verified. Samples were analysed in duplicate, and a difference ≤5 % was acceptable. Relative gene expression was calculated using the
![]() $2^{{{\minus}\Delta \Delta C_{t} }} $
method(
Reference Livak and Schmittgen
34
) with normalisation against the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase, as described in our previous study(
Reference Tan, Applegate and Liu
6
).
$2^{{{\minus}\Delta \Delta C_{t} }} $
method(
Reference Livak and Schmittgen
34
) with normalisation against the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase, as described in our previous study(
Reference Tan, Applegate and Liu
6
).
Table 3 Primers used in real-time quantitative PCR
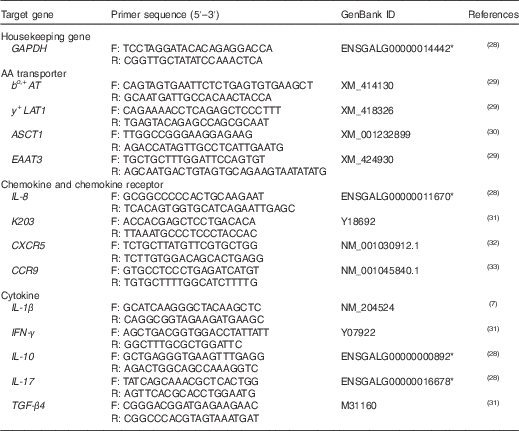
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; F, forward; R, reverse; bo,+AT, Na+-independent cationic and zwitterionic amino acid transporter; y+LAT1, Na+-independent cationic and Na+-dependent neutral amino acid transporter; ASCT1, system ASC, Na+-dependent neutral amino acid transporter 1; EAAT3, Na+, H+, K+-dependent excitatory amino acid transporter 3; CXCR5, C-X-C chemokine receptor type 5; CCR9, C-C chemokine receptor type 9; IL, interleukin; IFN-γ, interferon-γ; TGF-β4, transforming growth factor-β4.
* Sequence obtained from Ensembl chicken genome data resources.
Statistical analysis
All data (except for oocyst shedding) were analysed with two-way ANOVA using general linear model procedure of SAS (version 9.2; SAS Institute) for a completely randomised design. The model included main effects of dietary Thr concentration, feed withdrawal combined with coccidial infection and their interaction. Cage was the experimental unit, and treatment means were separated by least significant difference procedure. As the initial BW (13 d) was significantly affected by dietary Thr concentration, 13-d BW was used as a covariate in the model to analyse growth performance during challenge (13–21 d). For oocyst shedding under the combined challenge, contrast was used to separate the means with different dietary Thr concentrations. Differences with P≤0·05 were considered significant, and those with 0·05<P≤0·10 were considered a trend.
The partitioning of growth rate reduction under challenge was calculated as described by Pastorelli et al. ( Reference Pastorelli, van Milgen and Lovatto 35 ). In brief, the relationship between Δfeed intake (ΔFI) and ΔBW gain (ΔBWG) of challenged birds relative to unchallenged birds (expressed as percentage) for low-Thr or control Thr groups was analysed using quadratic regression: ΔBWG=α+β 1×ΔFI+β 2×ΔFI2. The analysis was carried out using the REG procedure in SAS. The intercept (α) represents ΔBWG related to changes in maintenance, which is not associated with ΔFI. The coefficients (β 1 and β 2) represent the extent of ΔBWG associated with ΔFI.
Results
Growth performance
Data on growth performance are presented in Table 4. The results show that bird performance was below that of the performance objectives of the primary breeder( 36 ), which is probably due to the lower hatching BW (33·8 (sem 0·2) g). In addition, these diets were formulated to be lower in crude protein, yet isonitrogenous with multiple crystalline amino acids supplemented back to achieve the differential in Thr per experimental design. Thus, performance up to the breeder optimal level would not be expected in this situation. At 13 d of age (before the challenge), Thr deficiency dramatically reduced 13-d BW by 21 % (P<0·0001). The interaction between dietary Thr concentration and combined challenge (feed withdrawal with coccidial vaccine overdose) was significant for 13–21-d BW gain (P=0·01). Under combined challenge, BW gain was dramatically decreased by 30 % (P≤0·05) and 26 % (P≤0·05) in the Thr-control group and the Thr-deficient group, respectively. In addition, the challenged group fed low Thr had 38 % lower BW gain (P≤0·05) compared with the Thr-control diet. However, no interaction was observed for 13–21-d FI and feed:gain (P>0·10). During the challenge period (13–21 d of age), both Thr deficiency and combined challenge significantly decreased FI and increased feed:gain (P≤0·004).
Table 4 Growth performance of broilers fed diets containing threonine concentrations of 4·9 or 9·0 g/kg with or without challenge from 13 to 21 d of age
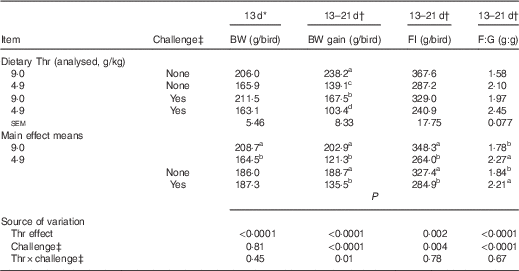
BW, body weight; FI, feed intake; F:G, feed:gain.
a,b,c,d Mean values within a column with unlike superscript letters were significantly different (P≤0·05).
* Means represent eight cages per treatment with seven birds per cage, analysed by ANOVA.
† Least square means represent eight cages per treatment with six birds per cage, analysed by ANCOVA with 13-d BW as the covariate.
‡ Challenge at 13–21 d: birds received 24-h feed withdrawal at 13 d of age with subsequent gavage of a 25× dose of coccidial vaccine.
In order to better understand the interaction effect of dietary Thr and challenge on BW gain, the partitioning of growth rate reduction following challenge for the low-Thr group or the control Thr group was calculated (Fig. 1 and 2). As shown in Fig. 1, the quadratic regressions between ΔBWG and ΔFI upon 13–21-d challenge were significant (P≤0·05; R 2≥0·85), regardless of dietary Thr concentration. The intercepts from both Thr groups were negative, suggesting that when ΔFI was not affected, the fraction due to change in maintenance contributing to ΔBWG was negative (Fig. 2). Therefore, under coccidial infection, birds from both Thr groups would have an increased maintenance requirement, and thus a lowered BWG. In addition, it was noticed that the fraction of change in maintenance for the Thr-deficient group (−18·7 %, Fig. 2) was almost 2-fold of that in the Thr-control group (−11·1 %, Fig. 2), indicating that Thr is a critical nutrient accounting for maintenance processes in coccidiosis.
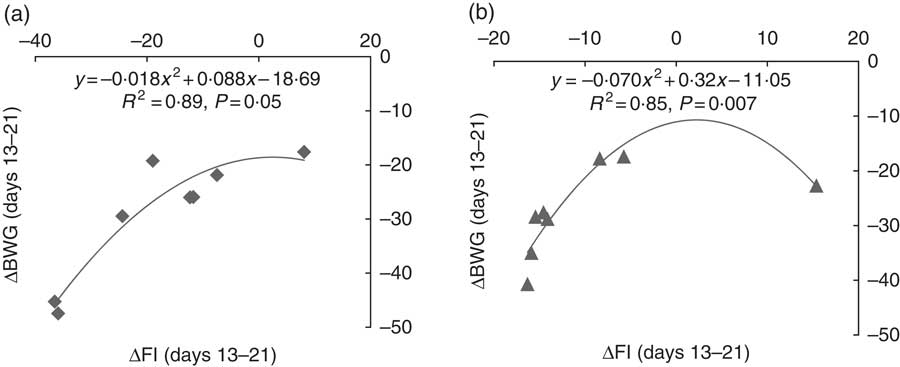
Fig. 1 Relationship between the change in BW gain (ΔBWG) and feed intake (ΔFI) of broiler chicks at 13–21 d of age challenged by feed withdrawal with vaccine overdose, when fed a low-Thr (a) or control-Thr (b) diet. Responses are expressed as results of the challenged birds relative to that of unchallenged birds fed the same diet. R 2, coefficient of correlation; P-value, level of significance for regression.
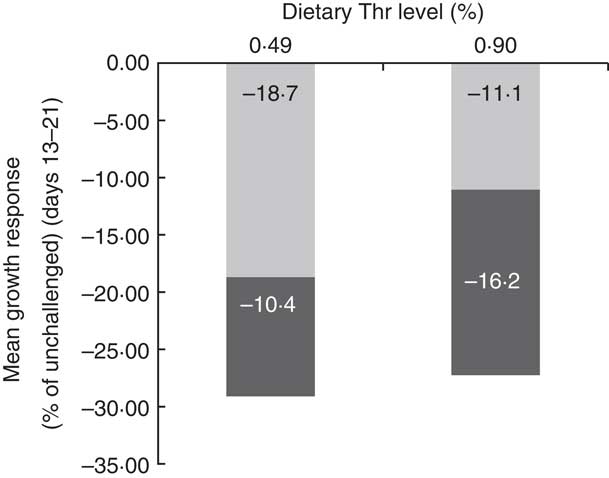
Fig. 2 Partitioning of the reduction in average BW gain of broiler chicks at 13–21 d of age challenged by feed withdrawal with vaccine overdose, between the fraction due to the change in maintenance (![]() , not associated with ΔFI) or change in feed intake (
, not associated with ΔFI) or change in feed intake (![]() , associated with ΔFI). ΔFI, Change in feed intake.
, associated with ΔFI). ΔFI, Change in feed intake.
Oocyst counting and gut lesion
The oocyst number was estimated in excreta at 21 d of age (7 d after coccidial vaccine challenge). No oocyst was detected in excreta from unchallenged birds, regardless of dietary Thr concentration. For the challenged birds, the analysis showed that Thr deficiency resulted in a higher number of oocysts in excreta (P=0·03, Table 5). No obvious lesions for the duodenum and the jejunum were observed, whereas bleeding in the caecum was occasionally detected regardless of the dietary Thr treatments, which indicated that birds in the present study had mild-to-moderate coccidial infection.
Table 5 Oocyst shedding in the excreta of broilers fed diets containing threonine concentrations of 4·9 or 9·0 g/kg with or without challenge at 21 d of age (Mean values with their standard errors; n 8 cages per treatment)
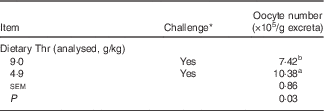
a,b Mean values within a column with unlike superscript letters were significantly different (P≤0·05).
* Challenge at 21 d: birds received 24-h feed withdrawal at 13 d of age with subsequent gavage of a 25× dose of coccidial vaccine.
Gut permeability and mucosa parameters
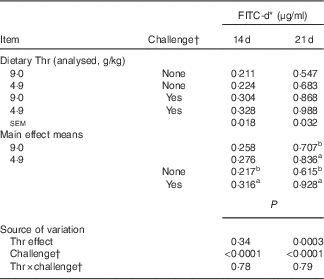
At both 14 and 21 d of age, gut permeability was measured by FITC-d passage into blood (Table 6), and the production of proteins related to intestinal barrier function, including mucins, IgA, IgM, pIgR/SC and claudin-1 from ileal mucosa, was determined (Tables 7 and 8).
Table 6 Serum fluorescein isothiocyanate-dextran (FITC-d) of broilers fed diets containing threonine concentrations of 4·9 or 9·0 g/kg with or without challenge at 14 and 21 d of age (Mean values with their standard errors; n 8 birds per treatment)
a,b Mean values within a column with unlike superscript letters were significantly different (P≤0·05).
* At 14 and 21 d of age, FITC-d (2·2 mg/bird, molecular weight 3000–5000) was orally gavaged to 1 bird/cage, and blood samples were collected 2 h after gavage.
† Challenge at 14 d: birds received 24-h feed withdrawal at 13 d of age (not gavaged with coccidial vaccine yet); challenge at 21 d: birds received 24-h feed withdrawal at 13 d of age with subsequent gavage of a 25× dose of coccidial vaccine.
Table 7 Mucin, IgA, IgM, polymeric Ig receptor/secretory component (pIgR/SC) and claudin-1 concentrations from ileal mucosa of broilers fed diets containing threonine concentrations of 4·9 or 9·0 g/kg with or without challenge at 14 d of age (Mean values with their standard errors; n 8 birds per treatment)
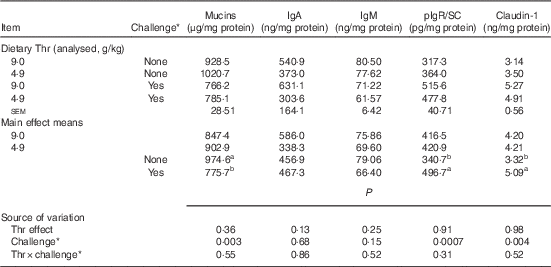
a,b Mean values within a column with unlike superscript letters were significantly different (P≤0·05).
* Challenge at 14 d: birds received 24-h feed withdrawal at 13 d of age (not gavaged with coccidial vaccine yet).
Table 8 Mucin, IgA, IgM, polymeric Ig receptor/secretory component (pIgR/SC) and claudin-1 concentrations from ileal mucosa of broilers fed diets containing threonine concentrations of 4·9 or 9·0 g/kg with or without challenge at 21 d of age (Mean values with their standard errors; n 8 birds per treatment)
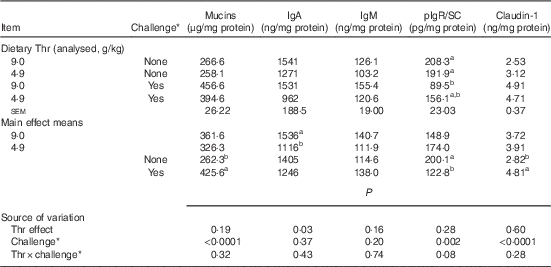
a,b Mean values within a column with unlike superscript letters were significantly different (P≤0·05).
* Challenge at 21 d: birds received 24-h feed withdrawal at 13 d of age with subsequent gavage of a 25× dose of coccidial vaccine.
Our results showed that at 14 d of age no interaction between dietary Thr concentration and challenge (24 h feed withdrawal) was detected based on serum FITC-d levels and concentrations of mucins, IgA, IgM, pIgR/SC and claudin-1 in ileal mucosa (P>0·10). In addition, dietary Thr concentration had no effect on all the aforementioned intestinal barrier function measures (P>0·10). However, 24-h feed withdrawal significantly increased serum FITC-d levels (P<0·0001), indicating higher gut permeability. Coincidently, with feed withdrawal, mucin production estimated from Alcian blue binding was highly decreased (P=0·003), whereas the production of pIgR/SC and claudin-1 was considerably increased (P≤0·004). Concentrations of IgA and IgM was not influenced by feed withdrawal (P>0·10).
At 21 d of age, no interaction between dietary Thr concentration and combined challenge (feed withdrawal with coccidial vaccine overdose) was observed for serum FITC-d levels and concentrations of mucins, IgA, IgM and claudin-1 in ileal mucosa (P>0·10). For the main effect, either Thr deficiency or feed withdrawal combined with vaccine overdose significantly increased gut permeability (P≤0·0003). Combined challenge increased mucin secretion and claudin-1 protein concentration (P<0·0001), but dietary Thr concentration had no effect on these two parameters. On the contrary, combined challenge had no influence on IgA concentration, but the Thr-deficient diet dramatically decreased IgA production (P=0·03). IgM concentration was not impacted by either Thr deficiency or combined challenge. A tendency to have significant interaction between the two factors was detected for the production of pIgR/SC in ileal mucosa (P=0·08). The combined challenge significantly decreased the production of pIgR/SC in birds fed the Thr-control diet (P≤0·05), but not in birds fed the Thr-deficient diet (P>0·05).
Jejunum morphology
At 21 d of age, jejunum morphology was determined (Table 9). Epithelial sloughing was observed in challenged birds. The interaction between dietary Thr concentration and combined challenge (feed withdrawal with coccidial vaccine overdose) had a tendency to be significant for VH (P=0·07). Although there was no difference in VH (P>0·05) between the challenged birds and the control regardless of dietary Thr concentration, Thr deficiency significantly decreased VH by 37 % (P≤0·05) and 45 % (P≤0·05) for control birds and challenged birds, respectively. A significant interaction between the two factors was observed for CD (P=0·03) and VH:CD ratio (P=0·05). For CD, combined challenge dramatically increased it to 2·3-fold (P≤0·05) and 1·9-fold (P≤0·05) in the Thr-control group and the Thr-deficient group, respectively. In addition, the challenged group fed low Thr had 31 % lower CD (P≤0·05), compared with the challenged group fed the Thr-control diet. For VH:CD ratio, combined challenge markedly decreased it by 53 % (P≤0·05) and 51 % (P≤0·05) in birds fed the Thr-control diet and birds fed the Thr-deficient diet, respectively. Moreover, the unchallenged birds fed the Thr-deficient diet had 26 % lower VH:CD ratio (P≤0·05), compared with the unchallenged birds fed the Thr-control diet. No interaction was observed for GC counts and GC density (P>0·10). Thr deficiency induced a decrease in GC counts (per villus, P<0·0001) but an increase in GC density (per µm of VH, P=0·008). Combined challenge had no effect on GC counts (per villus, P>0·10) but increased GC density (per µm of VH, P=0·04).
Table 9 Jejunum morphology of broilers fed diets containing threonine concentrations of 4·9 or 9·0 g/kg with or without challenge at 21 d of age (Mean values with their standard errors; n 8 birds per treatment)
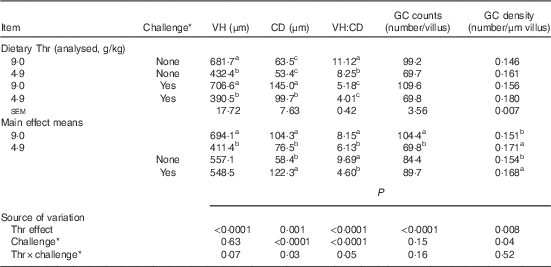
VH, villus height; CD, crypt depth; GC, goblet cells.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P≤0·05).
* Challenge at 21 d: birds received 24-h feed withdrawal at 13 d of age with subsequent gavage of a 25× dose of coccidial vaccine.
Gene expression of amino acid transporter
As shown in Table 10, at 21 d of age, no interaction between dietary Thr concentration and combined challenge (feed withdrawal with coccidial vaccine overdose) was observed for the expression of amino acid transporters. Combined challenge significantly decreased the mRNA expressions of Na+, H+, K+-dependent excitatory amino acid transporter 3 (EAAT3) and Na+-independent cationic and zwitterionic amino acid transporter (bo,+AT) (P≤0·002), but dietary Thr concentration had no effect on these variables (P>0·10). System ASC, Na+-dependent neutral amino acid transporter 1 (ASCT1) mRNA expression tended to increase with either Thr deficiency or combined challenge (P≤0·10).
Table 10 Relative gene expressionFootnote * of amino acid transporters in the jejunum of broilers fed diets containing threonine (Thr) concentrations of 4·9 or 9·0 g/kg with or without challenge at 21 d of age (Mean values with their standard errors; n 8 birds per treatment)
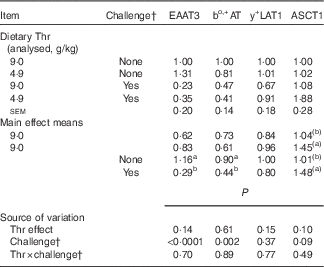
EAAT3, Na+, H+, K+-dependent excitatory amino acid transporter 3; bo,+AT, Na+-independent cationic and zwitterionic amino acid transporter; y+LAT1, Na+-independent cationic and Na+-dependent neutral amino acid transporter; ASCT1, system ASC, Na+-dependent neutral amino acid transporter 1.
a,b Mean values within a column with superscript letters were significantly different (P≤0·05).
(a),(b) Mean values within a column with superscript letters have a tendency to differ (0·05<P≤0·10).
* Relative gene expression (
![]() $2^{{{\minus}\Delta \Delta C_{t} }} $
)±SEM was calculated with glyceraldehyde-3-phosphate dehydrogenase as the endogenous control.
$2^{{{\minus}\Delta \Delta C_{t} }} $
)±SEM was calculated with glyceraldehyde-3-phosphate dehydrogenase as the endogenous control.
† Challenge at 21 d: birds received 24-h feed withdrawal at 13 d of age with subsequent gavage of a 25× dose of coccidial vaccine.
Lymphocyte profile
The difference in lymphocyte subpopulations of CT was assessed at 21 d of age. As presented in Table 11, no interaction between dietary Thr concentration and combined challenge (feed withdrawal with coccidial vaccine overdose) was observed for the percentage of lymphocytes expressing cell-surface antigens of CD4 (helper T cells), CD8 (cytotoxic T cells), CD3 (total T cells) and Bu-1 (total B cells). Thr deficiency resulted in a lower percentage of lymphocytes expressing CD4, CD8, CD3 and Bu-1 (P≤0·01), whereas the combined challenge increased the proportion of all aforementioned lymphocyte classes (P≤0·05). A tendency to have significant interaction between the two factors was observed for CD3:Bu-1 ratio (P=0·09). Under combined challenge, the CD3:Bu-1 ratio in the Thr-deficient group was significantly decreased (P≤0·05) compared with the Thr-control group, which was mainly because the low-Thr group under combined challenge had a lower increase in CD3 lymphocytes (5·33 %) compared with Bu-1 lymphocytes (10·8 %). In addition, the opposite evolution in the Thr-control group under combined challenge (+41 % for CD3 lymphocyte and +29 % for Bu-1 lymphocytes) contributed to its lower CD3:Bu-1 ratio. For the CD4:CD8 ratio, neither the interaction between the two factors nor the effect of Thr deficiency or combined challenge was observed.
Table 11 Percentage of lymphocytes expressing cell-surface antigens of CD4, CD8, CD3 and Bu-1 in caecal tonsil of broilers fed diets containing threonine concentrations of 4·9 or 9·0 g/kg with or without challenge at 21 d of age (Mean values with their standard errors; n 8 birds per treatment)
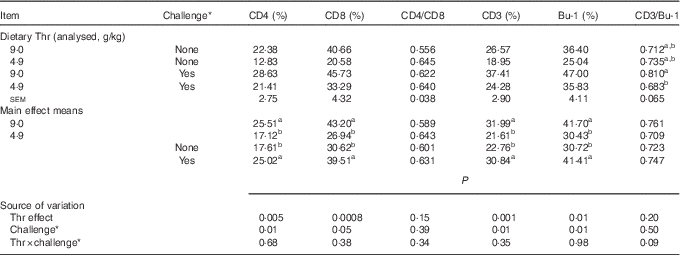
a,b Mean values within a column with unlike superscript letters were significantly different (P≤0·05).
* Challenge at 21 d: birds received 24-h feed withdrawal at 13 d of age with subsequent gavage of a 25× dose of coccidial vaccine.
Gene expressions of chemokines, chemokine receptors and cytokines
The mRNA expressions of chemokines, chemokine receptors and cytokines in CT at 21 d of age are presented in Table 12. No interaction between dietary Thr concentration and combined challenge (feed withdrawal with coccidial vaccine overdose) was detected for the expressions of assessed genes (except interferon-γ (IFN-γ)) in CT (P>0·10). Thr deficiency increased mRNA expressions of IL-17 and transforming growth factor-β4 (TGF-β4) (P≤0·008), and had a tendency to increase mRNA expressions of IL-10, K203 and CXCR5 (P≤0·10), but had a tendency to decrease CCR9 mRNA expression (P=0·10). Combined challenge increased mRNA expressions of IL-10, K203 and CCR9 (P≤0·02), and had a tendency to increase IL-8 mRNA expression (P=0·07), but decreased CXCR5 mRNA expression (P=0·03). A tendency to have significant interaction between the two factors was observed for IFN-γ mRNA expression (P=0·06). Under combined challenge, IFN-γ mRNA expression was significantly increased (P≤0·05) in birds fed the Thr-control diet, but the increase was not significant for the birds fed the Thr-deficient diet (P>0·05).
Table 12 Relative gene expressionsFootnote * of chemokines, chemokine receptors and cytokines in caecal tonsil of broilers fed diets containing threonine concentrations of 4·9 or 9·0 g/kg with or without challenge at 21 d of age (Mean values with their standard errors; n 8 birds per treatment)
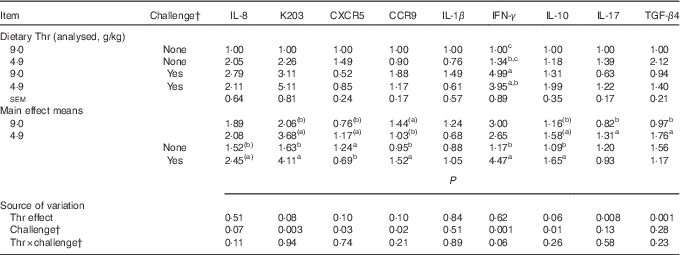
IFN-γ, interferon-γ; TGF-β4, transforming growth factor-β4; IL, interleukin; CXCR5, C-X-C chemokine receptor type 5; CCR9, C-C chemokine receptor type 9.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P≤0·05).
(a),(b) Mean values within a column with unlike superscript letters have a tendency to differ (0·05<P≤0·10).
* Relative gene expression (
![]() $2^{{{\minus}\Delta \Delta C_{t} }} $
)±sem was calculated with glyceraldehyde-3-phosphate dehydrogenase as the endogenous control.
$2^{{{\minus}\Delta \Delta C_{t} }} $
)±sem was calculated with glyceraldehyde-3-phosphate dehydrogenase as the endogenous control.
† Challenge at 21 d: birds received 24-h feed withdrawal at 13 d of age with subsequent gavage of a 25× dose of coccidial vaccine.
Discussion
Clinical symptoms of a coccidial infection include intestinal mucosal damage, poor feed efficiency and growth depression( Reference Lillehoj, Min and Dalloul 1 – Reference Shivaramaiah, Barta and Hernandez-Velasco 3 ). All of these symptoms are economically significant to the poultry industry, and the symptoms vary from mild to severe, depending on the levels of parasite pathogenesis in the intestinal mucosa( Reference Shivaramaiah, Barta and Hernandez-Velasco 3 ). In order to protect against Eimeria parasites, multiple facets of the host immune system have evolved, including epithelium barrier functions, macrophages, lymphocytes and their secretions, in the form of antibodies and cytokines( Reference Lillehoj, Min and Dalloul 1 – Reference Shivaramaiah, Barta and Hernandez-Velasco 3 , Reference Tan, Applegate and Liu 6 , Reference Collier, Hofacre and Payne 8 ). Among the essential amino acids, Thr is particularly important for producing mucus and maintaining gut integrity( Reference Nichols and Bertolo 10 – Reference Hamard, Mazuraisc and Boudry 15 ), in addition to its potential effect on modulating immune response( Reference Zhang, Xu and Doster 14 , Reference Duval, Demangel and Munier-Jolain 16 – Reference Azzam, Zou and Dong 18 ). Dietary Thr deficiency may limit mucin synthesis, disrupt barrier function and restructure the immune cell profile that leads to damage of intestinal protection. Thus, it is of interest to explore the interaction between dietary Thr deficiency and coccidial infection on the aforementioned aspects.
As temporary feed withdrawal predisposed birds to later outbreak of enteric pathogen colonisation and shedding( Reference Burkholder, Thompson and Einstein 37 ), 24-h feed withdrawal was applied at 13 d of age in the present study, which impaired the intestinal mucosal barrier, as evidenced by the higher gut permeability (as observed previously in young birds( Reference Vicuña, Kuttappan and Tellez 24 )) and lower mucin content (consistent to the observed reduction of the mucus content in birds at 39 d of age( Reference Thompson and Applegate 20 )), but surprisingly higher claudin-1 and pIgR/SC production from ileal mucosa. Claudin-1 is one of the TJ proteins in regulating paracellular barrier permeability( Reference Kansagra, Stoll and Rognerud 38 ). The major function of pIgR, expressed on the basolateral surface of intestinal epithelial cells, is to transport IgA (and to a lesser extent IgM) across the epithelial cells to the gut lumen. At the apical surface, cleavage of the extracellular portion of pIgR results in the release of SC as part of the secretory Ig complex or in a free form( Reference Kaetzel 39 , Reference Zhang, Eicher and Applegate 40 ). The seemingly contradictory increase in claudin-1 and pIgR/SC in this case suggested a possible compensatory role in the damaged mucosa barrier( Reference Kansagra, Stoll and Rognerud 38 , Reference Poritz, Harris and Kelly 41 , Reference Johansen and Kaetzel 42 ). Moreover, the change in other TJ proteins may also contribute to the increase in gut permeability, and more studies are warranted regarding this aspect. Mucin is a major component of the mucus layer, which has the effect to interact with Eimeria oocysts to inhibit their invasion of the intestinal epithelium( Reference Tierney, Matthews and Carrington 19 ). The dramatic reduction of mucin along with the increased gut permeability would make the birds more prone to coccidial infection. In addition, the coccidial infection induces a host mucogenic response( Reference Collier, Hofacre and Payne 8 ), and the pre-existent mucin reduction with feed withdrawal would drive more available Thr for mucin synthesis under coccidial infection to make the Thr-deficient condition more severe.
Dietary Thr deficiency had no effect on intestinal barrier parameters after 24 h of feed withdrawal. However, after coccidial vaccine overdose, Thr deficiency significantly increased gut permeability with a lower IgA production from ileal mucosa. It is likely that mucin synthesis has a priority over IgA production for Thr utilisation, as Thr deficiency had no effect on mucin content regardless of coccidial infection. Surprisingly, the increased pIgR/SC concentration after feed withdrawal was decreased under coccidial infection with the Thr-control diet, which may be attributed to the consumption of SC during the elimination of Eimeria parasites, because SC itself has direct scavenger properties against pathogen invasion( Reference Phalipon and Corthésy 43 ). However, the relatively higher pIgR/SC concentration for Thr-deficient group upon coccidial infection may be attributed to the lower IgA production (62·8 % of the Thr-control group upon infection), which leads to less binding with pIgR.
Under histological assessment, VH in challenged birds was not different from the control birds regardless of dietary Thr level. This was likely attributed to the challenge resulting in only a mild-to-moderate coccidiosis. Crypt elongation was detected in birds with coccidial infection, as it was previously observed in other studies( Reference Fernando and McCraw 4 – Reference Tan, Applegate and Liu 6 ). The longer CD indicated greater intestinal epithelial proliferation( Reference Chen, Tellez and Richards 44 ), which may represent a plausible mechanism for intestinal oocyst clearance and intestinal repair, as the infected birds with deficient Thr had higher oocyst shedding (+40 %) but lower CD (−31 %). However, more studies are warranted before drawing firm conclusions on this plausible mechanism. The lowest jejunal VH and VH:CD were observed in the coccidial vaccine-infected birds fed the low-Thr diet, indicating decreased nutrient absorption and increased cost to maintain gut integrity( Reference Chen, Tellez and Richards 44 ), and thus contributing to the lowest BW gain for infected birds under Thr deficiency. In addition, increased GC density in the jejunum was noticed with low-Thr diet, which was consistent with the results by Wils-Plotz & Dilger( Reference Wils-Plotz and Dilger 45 ), suggesting a possible compensatory role for Thr deficiency in increasing GC density. Correspondingly, the combined challenge increased the GC density as well, probably due to the aforementioned Thr-deficient condition induced by feed withdrawal.
In addition, the gene expressions of Thr-related amino acid transporters, bo,+AT, Na+-independent cationic and Na+-dependent neutral amino acid transporter (y+LAT1) and ASCT1, as well as the glutamate transporter EAAT3, were determined because of the potential that they might be affected by coccidial infection and/or feed withdrawal. The bo,+AT/rBAT transporter is located at the brush-border membrane and exchanges extracellular cationic amino acids (such as Lys and Arg) and Cys for intracellular neutral amino acids( Reference Paris and Wong 30 , Reference Su 46 ). The y+LAT1/4F2hC transporter is located at the basolateral membrane and exchanges neutral amino acids for intracellular cationic amino acids( Reference Su 46 ). ASCT1 is a transporter for small neutral amino acids such as Ala, Ser, Cys and Thr, but its location in chickens remains to be determined( Reference Paris and Wong 30 , Reference Su 46 ). In the present study, coccidial infection decreased bo,+AT and EAAT3 mRNA expressions, but had a tendency for increased ASCT1 mRNA expression. This is consistent with a previous analysis of the expression of these transporters in either the mucosa layer( Reference Paris and Wong 30 ) or the whole tissue of birds( Reference Su 46 ). The coccidia-induced down-regulation of bo,+AT and EAAT3 may result in decreased influx of Lys, Arg and the energy source glutamate, but increased uptakes of intracellular neutral amino acids including Thr. Up-regulation of ASCT1 in the Thr-deficient birds with infection further demonstrated that coccidiosis may necessitate an availability of sufficient Thr in the intestine.
Reduction in lymphocyte populations in the CT represents a weakened intestinal immune system with increased susceptibility to coccidial infection. In the present study, Thr-deficient birds exhibited fewer measured subpopulations of lymphocytes than birds fed an adequate level of Thr. Although the CD4:CD8 ratio was not affected by Thr levels, CD3:Bu-1 ratio in the Thr-deficient group was significantly lower than the Thr-control group under combined challenge, which was mainly due to a limited increase in the CD3 subpopulation (49 % of Bu-1 lymphocytes) upon coccidial infection. To our knowledge, this is the first study to demonstrate a Thr effect on lymphocyte subpopulations. The relatively lower increase in CD3 lymphocytes with coccidiosis in the Thr-deficient birds is probably another factor accounting for the higher oocyst shedding, because T cell-mediated immunity plays a central role in the control of intracellular Eimeria parasites( Reference Lillehoj, Min and Dalloul 1 – Reference Shivaramaiah, Barta and Hernandez-Velasco 3 ).
Failure to recruit T cells in Thr-deficient birds may suggest a disruption in recruitment signals to direct immune cell migration to sites of infection. Therefore, the gene expressions of chemokines IL-8 and K203 and chemokine receptors CXCR5 and CCR9 were determined in CT. IL-8 belongs to CXC chemokines, and its primary function is to recruit and activate neutrophils in response to infection( Reference Wigley and Kaiser 47 ). K203 is a CC chemokine that attracts macrophages and monocytes( Reference Walston 48 ). CCR9 in mammals is predominantly present on gut-homing T lymphocytes, and is also expressed on a subset of B and natural killer cells, to induce the formation of gut lymphoid tissue( Reference Annamalai and Selvaraj 33 , Reference Papadakis, Prehn and Nelson 49 ). CXCR5 in mammals is expressed on B cells and B-helper T cells and is necessary for the formation of intestinal humoral immunity( Reference Annamalai and Selvaraj 32 , Reference Ebert, Schaerli and Moser 50 ). In the present study, Thr deficiency had no effect on IL-8 mRNA levels, but induced a tendency for increased K203 and CXCR5 mRNA expressions and decreased CCR9 mRNA, indicating a trend towards a shift in the immune cells towards more B cells and macrophages than T cells under limited Thr intake. This would be consistent with the CD3:Bu-1 ratio from lymphocyte subpopulations. Therefore, the changes in chemokine signals could have contributed to an alteration in immune cell profiles.
Subsequently, the changed immune cell profiles may impair local immune responses in coccidiosis, through their secretion of cytokines. IL-1β is a pro-inflammatory cytokine, and its main function is to activate a range of cells including macrophages and T cells, which may lead to production of other cytokines and chemokines( Reference Wigley and Kaiser 47 ). Expression of IL-1β mRNA in this study was not affected by either Thr deficiency or coccidial infection, even though previous studies have shown that E. tenella induced higher IL-1β expression in the caecum( Reference Hong, Lillehoj and Lee 31 , Reference Laurent, Mancassola and Lacroix 51 ). The production of IFN-γ, which supports a Th1 profile, is crucial in the resistance to Eimeria ( Reference Lillehoj, Min and Dalloul 1 , Reference Dalloul and Lillehoj 2 ). IFN-γ, produced primarily by CD4 cells (rather than CD8 cells) after Eimeria infection( Reference Yun, Lillehoj and Choi 52 ), could directly inhibit the development of Eimeria sporozoite( Reference Lillehoj and Choi 53 ), augment cytotoxic activity of CD8 cells and enhance macrophage activation( Reference Lillehoj, Min and Dalloul 1 , Reference Schoenborn and Wilson 54 ). Further, it has been shown that IFN-γ administered alone or with a coccidial vaccine could induce an anti-coccidial immune response by reducing oocyst production and BW loss( Reference Lillehoj and Choi 53 , Reference Ding, Lillehoj and Quiroz 55 ). Previous studies have shown that IFN-γ mRNA is drastically increased in CT after exposure to E. tenella ( Reference Hong, Lillehoj and Lee 31 , Reference Laurent, Mancassola and Lacroix 51 ). In the present study, upon coccidial infection, IFN-γ mRNA expression was significantly increased in birds fed the Thr-control diet, and was also increased in the Thr-deficient group, although not significantly. These results are consistent with previous observations that Eimeria-susceptible lines of chicken had lower expression of IFN-γ during infection compared with resistant lines( Reference Yun, Lillehoj and Choi 52 ). IL-10 was initially described as a Th2 cytokine, but further evidence suggested that the production of IL-10 was associated with Treg cell response( Reference Walston 48 ). Rothwell et al. ( Reference Rothwell, Young and Zoorob 56 ) suggested that IL-10 has the capacity to prevent IFN-γ, driving anti-coccidial response, as evidenced by its inhibition of IFN-γ synthesis in mitogen-activated lymphocytes in vitro. Similarly, in vivo, it was reported that susceptible birds had up-regulated IL-10 mRNA expression after Eimeria infection, whereas resistant birds or susceptible birds treated with anti-coccidial drugs did not( Reference Rothwell, Young and Zoorob 56 , Reference Haritova and Stanilova 57 ). In our study, Thr-deficient birds had a higher IL-10 mRNA transcription, accompanying the reduced increase in IFN-γ mRNA expression under coccidial infection. These results support a possibility that lower IFN-γ and higher IL-10 may increase susceptibility to coccidiosis, as Thr-deficient birds had higher oocyst shedding and lower BW gain. Therefore, it seems that there is a reciprocal relationship between IFN-γ and IL-10 in coccidial immune response, and the outcome of pathogenesis depends on which cytokine dominates in infection progression. The role of the recently discovered IL-17, from the most recently described group of Th17 cells, is controversial in coccidial infection studies( Reference Ding, Lillehoj and Quiroz 55 , Reference Zhang, Liu and Song 58 ). Ding et al. ( Reference Ding, Lillehoj and Quiroz 55 ) demonstrated that IL-17 could enhance the protective effect of coccidial vaccine. However, Zhang et al. ( Reference Zhang, Liu and Song 58 ) reported that multiple injections of IL-17 until 8 d after infection enhanced faecal oocyst shedding and caecal lesion scores, probably due to the role of IL-17 in recruiting too many neutrophils at the sites of infection. The role of Th17 cells in both protective and pathologic roles during parasitic diseases still remains to be elucidated, but the signalling of Th17 requires cooperation of TGF-β4, which promotes the differentiation of naive T cells to the Th17 phenotype( Reference Zhang, Liu and Song 58 , Reference Konkel and Chen 59 ). In the present study, a higher IL-17 mRNA expression was coincided with an increased TGF-β4 mRNA abundance in birds fed low Thr. The increased IL-17 mRNA expression may induce an influx of neutrophils that damage the mucosal epithelium. In addition, TGF-β4 has an integral role in promoting Treg generation, which in turn secretes higher TGF-β4 and IL-10 to suppress the activation and proliferation of CD4 and CD8 T cells( Reference Walston 48 , Reference Konkel and Chen 59 ). Therefore, the increased IL-10 mRNA expression in Thr-deficient birds may be driven by the higher TGF-β4 mRNA abundance in those birds. Overall, the low-Thr diet could have led to increased TGF-β4 mRNA level, which may drive a higher mRNA expression of IL-10 to inhibit the IFN-γ mRNA transcription, eventually leading to a higher coccidiosis susceptibility. In addition, there is a possibility that increased expression of TGF-β4 mRNA in Thr-deficient birds could stimulate increased IL-17 mRNA transcription, leading to attraction of large numbers of neutrophils, resulting in mucosal damage and destruction.
In conclusion, birds fed the low-Thr diet showed more Eimeria oocyst shedding than birds fed diets with recommended levels of Thr, indicating a higher susceptibility to coccidiosis. Two potential mechanisms might explain this observation. First, impaired integrity of the intestinal barrier, mainly induced by the decreased IgA production and lowered crypt proliferation, could lead to increased invasion and replication of Eimeria parasites in the intestinal epithelium. Second, weakened local immune defences, characterised by the reduction in lymphocyte subpopulations in the CT, especially the lower content of T cells upon coccidial infection, could result in failure to mount an effective immune response against parasites. T cells are the primary components of protective immunity against Eimeria parasites, because they are responsible for orchestrating many other cellular immune responses through secretion of a host of cytokines such as IFN-γ, IL-10, IL-17 and TGF-β4. In addition, Thr deficiency notably decreased 13–21 d BW gain in birds with coccidiosis. Fractional analysis of average growth response under infection revealed that BW gain reduction induced by Thr deficiency was largely attributed to an increased maintenance requirement in support of the increased need for mucosal repair and immune activation. Therefore, further experiments are needed to evaluate the possibility of a high-Thr requirement in chickens during coccidiosis. The present study supports the role of an adequate dietary Thr level for optimal growth and immune response during coccidial infection.
Acknowledgements
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
The authors’ contributions are as follows: Q. Z. and T. J. A. were responsible for the study design; Q. Z. conducted the animal trial, performed the sample analyses and prepared the manuscript; X. C., S. D. E., K. M. A. and T. J. A. contributed to the sample analyses, reviewed and edited the manuscript. All the authors read and approved the final version of the manuscript.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. US Department of Agriculture is an equal opportunity employer.
None of the authors has any conflicts of interest to declare.



















