Type 2 diabetes (T2D) is a complex, heterogeneous and polygenic disease characterised mainly by insulin resistance and pancreatic β-cell dysfunction( Reference Salas-Salvado, Martinez-Gonzalez and Bullo 1 ). Impaired postprandial insulin secretion, because of functional defects and loss of pancreatic β-cells, leads to hyperglycaemia and subsequent decline in insulin sensitivity( Reference Aziz and Wheatcroft 2 ). Hyperglycaemia and hyperlipidaemia that accompanied T2D are key promoters of dysmetabolism( Reference Pérez-Matute, Zulet and Martínez 3 ).
Relationships between diabetes and diet composition have been shown in several humans and animals studies. Data reported that the type of fats in the diet may affect insulin resistance in animals( Reference Dobbins, Szczepaniak and Myhill 4 ). Rodents fed a high-fat diet (HFD) developed impaired intracellular glucose metabolism within 4 weeks. These animals also showed visceral obesity, whole-body and skeletal muscle insulin resistance, hyperinsulinaemia, hyperglycaemia and hyperlipidaemia( Reference Petrescu, Cheema and Fan 5 ).
In recent years, much focus has been placed on the beneficial effects of fish consumption, which many of positive effects on dyslipidaemia and heart diseases have been attributed to n-3 PUFA contained in fish oils( Reference Goldberg and Sabharwal 6 ). However, authors have previously demonstrated that protein component may also have an important role in the beneficial effects of fish consumption( Reference Tremblay, Lavigne and Jacques 7 , Reference Ouellet, Marois and Weisnagel 8 ). In this case, Lavigne et al.( Reference Lavigne, Tremblay and Asselin 9 ) have reported that cod protein in a high-fat, high-sucrose diet protected against the development of obesity-linked insulin resistance and glucose intolerance in rats, and it improved insulin sensitivity in insulin-resistant individuals( Reference Ouellet, Marois and Weisnagel 8 ). Moreover, a recent study showed that HFD combined with various fish proteins had anti-inflammatory effects and that salmon proteins protected against visceral obesity( Reference Pilon, Ruzzin and Rioux 10 ). Data have reported the importance of fish protein in the regulation of cholesterol metabolism( Reference Shukla, Bettzieche and Hirche 11 ). Moreover, studies have demonstrated the beneficial effect of sardine proteins (SP) on hyperglycaemia and hyperlipidaemia in streptozotocin (STZ)-induced diabetic rats( Reference Mellouk, AitYahia and Boukortt 12 ).
The aim of this study was to investigate the effects of SP on glycaemia, insulin resistance and reverse cholesterol transport, in T2D rats induced with an HFD (30 % lipids), associated with STZ injection.
Methods
Preparation of sardine proteins
Fish proteins isolated from sardine (Sardina pilchardus) fillets were previously described by Mellouk et al.( Reference Mellouk, AitYahia and Boukortt 12 ). Purified SP preparation constituted 92 % proteins, 0·2 % lipids and 4·8 % ashes.
Animals and diets
Male Wistar rats (n 30) (Pasteur Institute) weighing 260 (sem 25) g were maintained at stable temperature (22−23°C) and humidity (60 %), with 12 h light–12 h dark cycle (light 07.00–19.00 hours). This study was approved by our Institutional Animal Research Committee. The General Guidelines on the Use of Living Animals in Scientific Investigations Council of European Communities (1987) were followed( 13 ).
At the beginning of the experiment, rats were fed an HFD containing 20 % casein (CAS) and 30 % butter (containing 67 % SFA) for 5 weeks in order to induce insulin resistance. After this period, rats weighing 366 (sem 25) kg received a single intraperitoneal injection of low-dose STZ (35 mg/kg body weight (BW)) (Sigma) in 0·1 m-citrate buffer (pH 4·5) after an overnight fast. Diabetes was identified by measuring fasting glycaemia 48 h after STZ injection (glycaemia at d0=6·63 (sem 0·33) mm, and after 48 h STZ injection 24·36 (sem 4·66) mm). Animals with blood glucose levels above 11 mm were considered diabetic (n 24), and they were randomly divided into four groups of six rats each and fed for 28 d a diet containing 20 % CAS or purified SP, combined with 5 % of mixed oils (3·9 % olive, 1 % nut, 0·1 % sunflower, with a n-6:n-3 ratio of 7) (CAS, SP), or with 30 % lipids (CAS-HF, SP-HF). The diet composition is shown in Table 1. Food and water were provided ad libitum. Glycaemia (using a one-touch glycometer Accu-Chek Active; Accu-Chek) and BW were recorded weekly. Over a 5- d period during the 4th week, faecal and urinary samples were collected in both groups housed in metabolism cages.
Table 1 Composition of the experimental diet (g/kg)
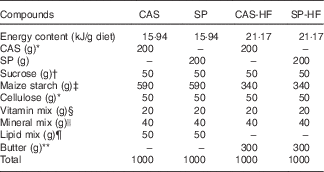
CAS, casein; SP, sardine proteins; HF, high fat.
* Prolabo.
† ENASUCRE.
‡ Sidi Bel Abbès.
§ UAR 200: vitamin mixture provides the following amounts (mg/kg diet): retinol, 12; cholecalciferol, 0·125; thiamine, 40; riboflavin, 30; pantothenic acid, 140; pyridoxine, 20; inositol, 300; cyanocobalamin, 0·1; ascorbic acid, 1·600; dl-α-tocopherol, 340; menadione, 80; nicotinic acid, 200; para-aminobenzoic acid, 100; folic acid,10; biotin, 0·6.
|| UAR 205 B: the salt mixture provides the following amounts (mg/kg diet): CaHPO4, 17 200; KCl, 4000; NaCl, 400; MgO, 420; MgSO4, 2000; Fe2O3, 120; Fe2SO4.7H2O, 200; trace elements, 400; MnSO4.H2O, 98; CuSO4.5H2O, 20; ZnSO4, 80; CoSO4.7H2O, 0·16; Kl, 0·32.
¶ Lipid mix: lipid mixture provides the following amounts (g/kg diet): sunflower oil, 10; olive oil, 39; walnut oil, 1 (with n-6:n-3=7). Olive and sunflower oils from Cevital; walnut oil from Cauvin.
** Butter, commercial butter from Oran.
Blood and liver samples
At d28, after overnight food deprivation, rats were anesthetised with pentobarbital 10 % (3 mg/kg BW). Blood was withdrawn from the abdominal aorta. Serum was obtained by low-speed centrifugation at 1000 g for 20 min at 4°C. Liver was removed immediately, rinsed with cold NaCl 0·9 %, dried and weighed. An aliquot of 100 mg of liver was stored at −70°C until analysis.
Biochemical analysis
The calculation of intra- and inter-assay imprecision (%CV) in the followed assays was between 10 and 15 %. In our experiment, for the insulin and serum glucose, the results showed a low variability of the data, indicating an homogeneous data, whereas for lecithin:cholesterol acyltransferase (LCAT) activity, particularly for SP and SP-HF groups, the %CV exceeds 15 %, reflecting a wide variability between the animals (heterogeneous data).
Serum glucose, insulin and glycosylated Hb
Serum glucose concentration was measured using a glucose oxidase (kit; Spinreact). Taking into account the variability of serum glucose levels in rats, glycosylated Hb (HbA1c) levels were used as an index of glucose control. HbA1c was measured by the cation-exchange resin (micro-column) method (kit; Biosystems). Serum insulin levels (kit; SPI-Bio) were determined and insulin resistance index estimated by the homeostasis model assessment insulin resistance (HOMA-IR) was calculated using relationships between blood glucose and insulin levels( Reference Matthews, Hosker and Rudenski 14 ), according to formula: HOMA-IR index=(fasting glucose (mmol/l)−fasting insulin (mU/ml))∕22·3. HOMA-β, which estimates the β-cell function rate, was calculated using the following equation: (20×fasting insulin (μU/ml)/(fasting glucose (mmol/l)−3·5))( Reference Matthews, Hosker and Rudenski 14 ).
Transaminases
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities, used as biochemical markers for hepatic damage, were determined using enzymatic methods (kits; Chronolab).
Lipoprotein separation
Serum VLDL and LDL-HDL1 were isolated by precipitation using the MgCl2/phosphotungstate method( Reference Burstein, Scholnick and Morfin 15 ). HDL2 and HDL3 subfractions were separated by the precipitation method( Reference Burstein, Fine and Atger 16 ), using dextran sulphate and MgCl2 (Sigma Chemical Company).
Lipid parameters
Liver total lipids were determined by the method of Delsal( Reference Delsal 17 ). TAG and total cholesterol (TC) were determined in serum, liver and lipoprotein fractions by enzymatic methods (kits; Biocon Diagnostik). Phospholipids (PL) were determined by the enzymatic method (kit; Cypress Diagnostics).
Determination of lecithin:cholesterol acyltransferase activity
LCAT activity was determined on fresh serum by the endogenous method( Reference Chen and Lacko 18 ), and it was based on disappearance of unesterified cholesterol (UC) molecules, which were transformed into esterified cholesterol (EC) after 4 h of incubation at 37ºC, starting from a fatty acid and lecithin. UC value was determined by the enzymatic method (kit CHOD-PAP; Wako Chemicals). LCAT activity was expressed as nanomoles of EC/h per ml of serum.
Statistical analysis
Results were expressed as means with their standard errors of six rats per group. Statistical analysis of data was determined using STATISTICA 4.1 (StatSoft). Data were tested by two-way ANOVA followed by non-parametric least significant difference test. Differences and effects were considered significant at P<0·05 – protein effect: SP v. CAS regardless of the lipid content in the diet, and lipids effect: 5 v. 30 % lipids regardless of the protein.
Results
Body weight, food and water intake, and urine volume
At d0, the BW of the rats was similar in all the groups, whereas, at d28, an increase by 20 % was observed in the SP-HF group compared with the CAS-HF group. In SP, CAS and SP-HF groups, a weight gain was noted, whereas a loss was shown in the CAS-HF group. There was no significant difference in food and water intake (Table 2). Urine volume was increased by 28 % in CAS-HF compared with CAS and decreased by 15 % in SP-HF compared with CAS-HF (Table 2).
Table 2 Body weight, food and water intake and urine volumeFootnote * (Mean values with their standard errors)
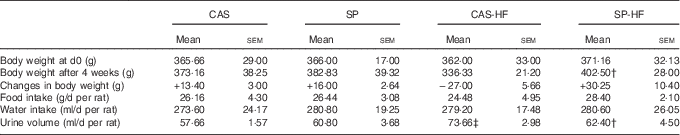
CAS, casein; SP, sardine proteins; HF, high fat.
* Statistical analysis was performed using the least significant difference test. The values were significantly different at P<0·05.
† SP v. CAS (P<0·05).
‡ SP-HF v. SP or CAS-HF v. CAS (P<0·05).
Glycaemia
At d7, d14, d21 and d28 of the experiment, glycaemia was increased by 26, 17, 49 and 135 %, respectively, in the CAS-HF group compared with the CAS group (Fig. 1). In contrast, glycaemia was lowered by 22, 26 and 33 % in SP compared with CAS at d14, d21 and d28 of the experiment, and by 23, 37, 43 and 67 % in SP-HF compared with CAS-HF at d7, d14, d21 and d28. HFD compared with the diet with 5 % lipids induced high glycaemia, regardless of the protein consumed (Fig. 1).

Fig. 1 Blood glucose concentrations in casein (CAS) (![]() ), sardine proteins (SP) (
), sardine proteins (SP) (![]() ), CAS-high diet (HF) (
), CAS-high diet (HF) (![]() ) and SP-HF (
) and SP-HF (![]() ) groups. Values are means (n 6), with their standard errors. Statistical analysis was performed using the least significant difference test. Mean values was significantly different (P<0·05). * SP v. CAS (P<0·05). † SP-HF v. SP or CAS-HF v. CAS (P<0·05).
) groups. Values are means (n 6), with their standard errors. Statistical analysis was performed using the least significant difference test. Mean values was significantly different (P<0·05). * SP v. CAS (P<0·05). † SP-HF v. SP or CAS-HF v. CAS (P<0·05).
Serum glucose, insulinaemia, glycosylated Hb, homeostasis model assessment insulin resistance, homeostasis model assessment β, aspartate aminotransferase, alanine aminotransferase activities and urine glucose
The HFD consumption induced an elevation of HOMA-IR, AST and ALT activities regardless of the consumed proteins, whereas HOMA-β index was reduced by 55 % in CAS-HF v. CAS. In SP v. CAS, a decrease was observed in serum glucose (64 %), HOMA-IR index (71 %), HbA1c (41 %), AST (17 %) and ALT (38 %) activities. Similarly, in SP-HF v. CAS-HF, a significant decrease was observed in serum glucose (37 %), HOMA-IR index (51 %), HbA1c (29 %), AST (27 %) and ALT (44 %). Moreover, serum insulin level lowered by 51 % in the SP group compared with the CAS group. In addition, HOMA-β was increased in rats fed SP regardless of the lipid content in the diet. Urine glucose was increased by 35 % in CAS-HF compared with CAS, whereas in SP v. CAS and SP-HF v. CAS-HF urine glucose concentrations were reduced by 28 and 40 %, respectively (Table 3).
Table 3 Serum biochemical parametersFootnote * (Mean values with their standard errors)
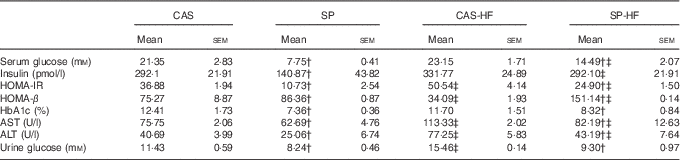
CAS, casein; SP, sardine proteins; HF, high fat; HOMA-IR, homeostasis model assessment insulin resistance; HbA1c, glycosylated Hb; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
* Statistical analysis was performed using the least significant difference test. The values were significantly different at P<0·05.
† SP v. CAS (P<0·05).
‡ SP-HF v. SP or CAS-HF v. CAS (P<0·05).
Serum, liver and faecal lipid concentrations
Serum TC and TAG concentrations were increased, respectively, by 48 and 101 % in CAS-HF v. CAS and by 32 and 86 % in SP-HF v. SP. Similarly, high values were noted in liver TC and TAG (76 and 48 %, respectively) in CAS-HF v. CAS and (30 and 21 %, respectively) in SP-HF v. SP (Table 4). Interestingly, these values decreased in SP compared with CAS, and in SP-HF compared with CAS-HF. Faecal total lipids were 2·08- and 2·22-fold higher, respectively, in SP than in CAS and in SP-HF than in CAS-HF. Indeed, faecal cholesterol concentrations were 1·58- and 1·53-fold higher, respectively, in the same groups.
Table 4 Serum, liver and faecal lipid concentrationsFootnote * (Mean values with their standard errors)
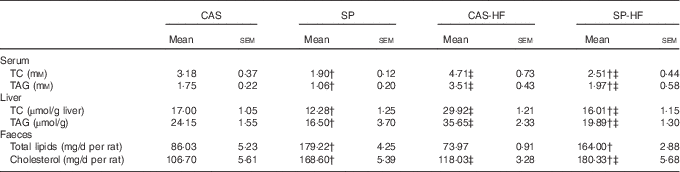
CAS, casein; SP, sardine proteins; HF, high fat; TC, total cholesterol.
* Statistical analysis was performed using the least significant difference test. The values were significantly different at P<0·05.
† SP v. CAS (P<0·05).
‡ SP-HF v. SP or CAS-HF v. CAS (P<0·05).
Total cholesterol and TAG concentrations in lipoproteins
In diabetic rats, HFD induced an increase in VLDL-cholesterol, VLDL- and LDL-TAG, whereas a decrease of HDL2-cholesterol was noted (Table 5). Moreover, HFD with CAS compared with CAS increased LDL-HDL1-cholesterol, HDL2- and HDL3-TAG by 43, 63 and 52 %, respectively. In the SP-HF group, HDL3-cholesterol was increased by 42 % compared with the SP group. In contrast, SP v. CAS combined with 5 or 30 % lipids induced a decrease of VLDL-cholesterol, LDL-HDL1-cholesterol, VLDL-TAG, LDL-HDL1-TAG, HDL2-TAG and HDL3-TAG. Moreover, in the SP v. CAS groups, HDL2-cholesterol enhanced by 50 %, whereas HDL3-cholesterol lowered by 37 %.
Table 5 Distribution of cholesterol and TAG in lipoproteinsFootnote * (Mean values with their standard errors)
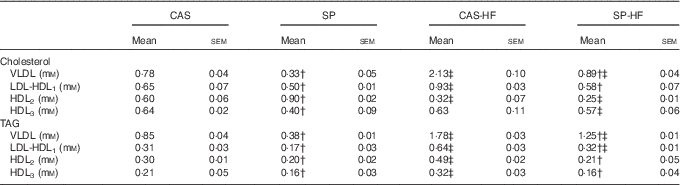
CAS, casein; SP, sardine proteins; HF, high fat.
* Statistical analysis was performed using the least significant difference test. The values were significantly different at P<0·05.
† SP v. CAS (P<0·05).
‡ SP-HF v. SP or CAS-HF v. CAS (P<0·05).
Lecithin:cholesterol acyltransferase activity
In the CAS-HF v. CAS groups, serum LCAT activity (Table 6) and its substrate HDL3-PL were 9·46- and 1·29-fold higher, respectively, whereas there was no significant difference in acyl acceptor HDL3-UC. The reaction product HDL2-cholesteryl esters (CE) was 3·55-fold lower. In the SP-HF group, HDL3-PL was 2·13-fold higher and HDL2-CE was 6·31-fold lower than in the SP group. However, in the SP group compared with the CAS group, serum LCAT activity, apoA-I and HDL2-CE were 4-, 1·97- and 3·15-fold higher, respectively, whereas HDL3-PL and HDL3-UC were 1·8- and 1·61-fold lower, respectively. In SP-HF compared with CAS-HF, LCAT activity, apoA-I and HDL3-UC concentrations were lower and HDL2-CE values were higher.
Table 6 Serum lecithin:cholesterol acyltransferase (LCAT) activity, apoA-I, HDL3-phospholipids (PL), HDL3-unesterified cholesterol (UC), HDL2-cholesteryl esters (CE) and apo-HDL3 Footnote * (Mean values with their standard errors)
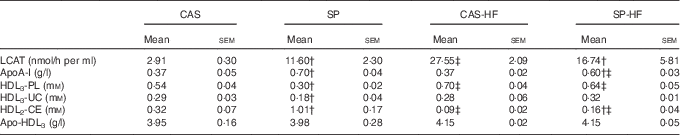
CAS, casein; SP, sardine proteins; HF, high fat.
* Statistical analysis was performed using the least significant difference test. The values were significantly different at P<0·05.
† SP v. CAS (P<0·05).
‡ SP-HF v. SP or CAS-HF v. CAS (P<0·05).
Discussion
The aim of the current work was to investigate the effects of SP on hyperglycaemia, insulin resistance, lipid parameters and LCAT activity, in rats fed HFD, followed by a single intraperitoneal low-dose (35 mg/kg BW) injection of STZ.
In diabetic rats fed HFD with CAS, glycaemia increased significantly, compared with that of rats fed a diet with 5 % lipids. In contrast, SP consumption significantly lowered glycaemia values in diabetic rats fed a 30 or a 5 % lipid diet, compared with CAS, from d7 of the experiment. These results are in agreement with those of other authors( Reference Lavigne, Tremblay and Asselin 9 ), who reported that fish protein consumption improved muscle sensitivity to insulin and increased glucose absorption in normoglycaemic rats. This phenomenon was also observed in obese rats( Reference Tremblay, Lavigne and Jacques 7 ), and in humans( Reference Ouellet, Marois and Weisnagel 8 ). The hypoglycaemic effect of SP could be attributed to the improvement of insulin action, by reducing glucose release from hepatocytes and stimulating glucose utilisation in peripheral tissue or increasing glycogenesis. Data reported that insulin:glucose ratio was lowered in subjects receiving cod protein, presenting smaller insulin excursion, when compared with those fed milk proteins( Reference von Post-Skagegârd, Vessby and Karlström 19 ). Furthermore, it was possible that SP-derived amino acids stimulated glucose uptake and utilisation in peripheral tissue. In this case, taurine is reported to control hyperglycaemia in alloxan-induced diabetic rats( Reference Joydeep, Vandana and Parames 20 ). As sardines are a much richer source of taurine( Reference Shirai, Terayama and Takeda 21 ), this may also explain why the SP rats displayed lower glucose, insulin and insulin resistance than the CAS-fed rats. In addition, oral administration of arginine to diabetic hamsters( Reference Popov, Costache and Georgescu 22 ) or intraperitoneal administration of arginine to rats with alloxan-induced diabetes( Reference Mendez and Balderas 23 ) reduced plasma glucose levels by up to 65 %. The mechanism was that arginine could stimulate the insulin secretion by pancreatic β-cells( Reference Flynn, Meininger and Haynes 24 ). Given the high arginine content in SP, this could strengthen our results, as an increase in HOMA-β indicating improved insulin secretory capacity of pancreatic β-cells was observed in SP v. CAS regardless of the fat content in the diet. The available evidence suggested that the action of insulin and arginine involved the following mechanisms. First, both insulin and arginine stimulated nitric oxide (NO) production by endothelial cells, which would contribute to increase the blood flow and, therefore, glucose and amino acid uptake by skeletal muscle in vivo ( Reference Flynn, Meininger and Haynes 24 ). Second, NO itself stimulated GLUT and oxidation by skeletal muscle. Third, physiological concentrations of NO might inhibit muscle proteolysis( Reference Flynn, Meininger and Haynes 24 ).
Rats fed HFD compared with a diet with 5 % lipids showed a significant decrease in glucose tolerance, HOMA-β and increase of HOMA-IR value. Dietary fat modification leads to changed cell membrane fatty acid composition, and it is associated with impaired insulin binding by decreasing the number of insulin receptors and GLUT. In addition, intracellular post-receptor disorders of liver, muscle (increased storage of TAG) and adipose tissue were observed( Reference Storlien, Baur and Kriketos 25 ). However, SP v. CAS showed increasing glucose tolerance given by the lower HOMA-IR value. Madani et al.( Reference Madani, Louchami and Sener 26 ) reported that SP decreased plasma insulin and insulin resistance. The improved insulin sensitivity observed with SP may be because of the amino acid composition. It was shown that taurine normalises glucose metabolism and attenuates hyperinsulinaemia in high-fructose-fed rats( Reference Nandhini and Anuradha 27 ); in addition, taurine levels were increased in rats fed SP compared with CAS( Reference Madani, Louchami and Sener 26 ).
During diabetes mellitus, HbA1c amount is greater. Diabetic rats fed SP compared with the CAS group showed low HbA1c level. Mellouk et al.( Reference Mellouk, AitYahia and Boukortt 12 ) reported a significant decrease in serum glucose and HbA1c in STZ-induced diabetic rats fed SP as compared with those fed CAS. It has been suggested that amino acid composition of SP could be responsible for different effects on HbA1c. Indeed, authors have shown that administration of taurine and glycine to diabetic rats significantly lowered the glucose content and HbA1c value, compared with untreated diabetic rats( Reference Alvarado-Vasquez, Zamudio and Ceron 28 ).
In addition, elevated biomarker enzymes, such as ALT and AST, were observed in diabetic rats, indicating liver damage induced by HFD. In contrast, SP showed a significant decrease in serum AST and ALT levels, indicating restored hepatic damage induced by diabetes. Thus, SP seem to have protective effects on the liver of diabetic rats.
Hyperlipidaemia has been reported to accompany hyperglycaemia state. Moreover, consumption of HFD is well known to cause increased chylomicron synthesis and absorption, which leads to increased TC and TAG levels, as well as endogenous VLDL production( Reference Jang, Srinivasan and Lee 29 ). In addition, increased hydroxy-methylglutaryl-CoA reductase and decreased lipoprotein lipase activities were attributed to HFD inducing dyslipidaemia( Reference Choi, Seog and Park 30 ).
In serum and liver, TC concentrations were higher with HFD, compared with the diet with 5 % fats. In contrast, SP consumption induced a hypocholesterolaemic effect, regardless of lipid amounts in the diet (30 or 5 % lipids). Indeed, in animals, dietary fish proteins reduced plasma cholesterol concentrations better than CAS( Reference Shukla, Bettzieche and Hirche 11 , Reference Mellouk, AitYahia and Boukortt 12 ). The hypocholesterolaemic response to SP may be ascribed to a decreased activity of cholesterol biosynthesis enzymes, as liver cholesterol content was low. This was consistent with the greater faecal lipids and cholesterol excretion. This mechanism could be because of increased bile acid synthesis, as reported by Liaset et al.( Reference Liaset, Madsen and Hao 31 ), who demonstrated that fish protein hydrolysates, rich in taurine and glycine, increased plasma bile acids levels concomitantly with reduced liver lipids. Furthermore, SP amino acid composition compared with CAS was different. Thus, in our previous study, fish proteins showed more arginine than CAS (6·5 v. 3·6 g/100 g proteins, respectively), and lysine:arginine ratio was lower in SP v. CAS (1·07 v. 2·05, respectively)( Reference Boukortt, Girard and Prost 32 ). Low lysine:arginine ratio( Reference Spielmann, Noatsch and Brandsh 33 ) and specific amino acid content, such as taurine, methionine, cysteine and glycine( Reference Shirai, Terayama and Takeda 21 ), might be responsible for this effect. In addition, taurine lowered TAG and TC levels in diabetic rats, and this was because of activated 7α-hydroxylase, which participates in biliary acid synthesis( Reference Nakaya, Minami and Harada 34 ). HDL2-cholesterol concentrations were higher in the SP group compared with the CAS group. Elevated serum HDL2-cholesterol is known to be protective against atherosclerosis development. These results are in agreement with those of other authors( Reference Lavigne, Tremblay and Asselin 9 , Reference Mellouk, AitYahia and Boukortt 12 ).
HFD feeding diabetic rats showed a significant increase in serum and liver TAG, whereas low serum and liver TAG concentrations were observed in rats fed the SP diet. This was probably because of a reduced TAG synthesis in the liver and their secretion in serum, LCAT and tissue lipases activation, as well as decreased fatty acid synthesis. These results are similar to those of others authors( Reference Mellouk, AitYahia and Boukortt 12 , Reference Murata, Sano and Bannai 35 ).
LCAT is a plasma enzyme that is responsible for the formation of most CE that are found in circulation( Reference Lima, Coelho and Kennedy 36 ). It has a key role in incorporating UC into HDL, and transferring back to VLDL or LDL, which is taken back by liver cells( Reference Savel, Lafitte and Pucheu 37 ). In the present study, LCAT activity and HDL3-PL were significantly higher in rats fed HFD v. 5 % lipids with CAS, whereas HDL2-CE were decreased. In diabetes and the metabolic syndrome, increased LCAT activity was noted, which was reflective of LCAT production( Reference Kappelle, de Boer and Perton 38 ). It has been demonstrated that this elevation did not predict low incidence of CVD( Reference Dullaart, Perton and van der Klauw 39 ), because it might modify the antioxidant and anti-inflammatory effects of HDL. Compared with the CAS-HF diet, the SP-HF diet lowered LCAT activity but increased the reaction product HDL2-CE, suggesting that SP were more efficient in this case.
In the SP group, LCAT activity and its cofactor-activator apoA-I increased, when compared with the CAS group. These results are in agreement with those of other authors( Reference Shukla, Bettzieche and Hirche 11 ), who found higher LCAT activity in rats fed fish proteins than in those fed CAS. In addition, HDL3-PL (substrate of LCAT) and HDL3-UC (acyl group acceptor) concentrations were significantly decreased, and HDL2-CE levels were increased. The enhancement of LCAT activity may be beneficial for normal HDL-cholesterol metabolism and may have a role in reverse cholesterol transport. Our results suggested that SP acted favourably on cholesterol efflux via HDL, when the lipid content and composition, in the diet, was normalised (i.e. 5 % lipids).
Nevertheless, this study concerning LCAT activity presented some limitations, and the results should be taken cautiously, knowing that the CV calculated showed a heterogeneity of the data, particularly for SP and SP-HF groups. Moreover, in diabetes mellitus, advanced glycation end products impair lipid metabolism by diminishing the expression of ABCA-1, ABCG-1 and the activity of LCAT. ABCG-1 has a major role in the efflux of 7-ketocholesterol to large HDL minimising its apoptotic effects in macrophages( Reference Iborra, Machado-Lima and Castilho 40 ). Thus, it could be interesting to clarify the mechanisms implicated in the reverse cholesterol transport, particularly the ABCA or ABCG transport proteins role in this diabetes type.
In conclusion, the present study suggests that an HFD, in diabetic rats, induces insulin resistance, hyperglycaemia and hyperlipidaemia. SP administration exerts an antihyperglycaemic effect and increases glucose tolerance. Moreover, SP have a hypocholesterolaemic effect by increasing faecal cholesterol excretion and hypotriglyceridaemic effect by decreasing liver synthesis of TAG. SP stimulate cholesterol efflux by enhancing LCAT activity. These effects seem to be more effective in diabetic rats fed a diet with 5 % lipids. Thus, SP may be considered as an applicable approach in preventing or treating diabetes and its associated complications.
Acknowledgements
This research was supported by the General Directorate for Scientific Research and Technological Development of the Ministry of Higher Education and Scientific Research.
N. B.: experimental study, sample collection, data interpretation and manuscript writing; F. Z. L.: experimental study and sample collection; M. B.: conception of the study, data interpretation and manuscript writing; F. O. B.: conception of the study, data analysis and interpretation, and manuscript writing.
The authors have no conflicts of interest regarding this article.












