The usefulness of microbial phytase in releasing phytate-bound phosphorus and improving phosphorus bioavailability in poultry diets is well documentedReference Cowieson, Hruby and Pierson1, Reference Selle and Ravindran2. In recent years, however, the positive effects, although to varying extents, on the apparent ileal amino acid (AA) digestibility of ingredients and complete poultry diets following phytase supplementation are also being increasingly recognisedReference Selle, Ravindran, Caldwell and Bryden3. Although the mode(s) of action underlying the AA digestibility responses to added phytase remain largely speculative, this effect may be explained, in part, by the amelioration of possible negative effects of phytic acid (PA) in increasing ileal endogenous AA flow. Endogenous proteins originate primarily from various digestive secretions, mucoproteins and sloughed epithelial cells lining the gut. It is known that the amounts of endogenous protein recovered at the distal ileum are increased by a number of anti-nutritive factors including trypsin inhibitors, tannins and lectinsReference Nyachoti, de Lange, McBride and Schulze4. Cowieson et al. Reference Cowieson, Acamovic and Bedford5, using a precision feeding assay, reported that PA increases and microbial phytase lowers the excretion of sialic acid, an endogenous compound associated with gastrointestinal mucin. An increased understanding of the mode of action of phytase in relation to AA would be useful to explain the variable responses in AA digestibility.
It is now recognised that the AA that disappear post-ileum are not beneficial to chickensReference Ravindran, Hew, Ravindran and Bryden6. Thus the endogenous AA flows determined at the ileal level represent true losses to the bird rather than those determined in the excreta. Different methods have been employed to estimate ileal endogenous AA flow in poultry, including feeding protein-free diets, regression method, homoarginine technique, peptide alimentation and the use of isotope markersReference Ravindran and Bryden7. The peptide alimentation technique described by Moughan et al. Reference Moughan, Darragh, Smith and Butts8 has been previously used to measure ileal AA losses in chickensReference Ravindran and Hendriks9, Reference Ravindran, Hew, Ravindran and Bryden10. The peptide alimentation method represents an improvement over the traditional protein-free diet method for the measurement of endogenous protein flow, in that endogenous losses are determined under normal feeding and physiological conditions. In this method, the animal is fed a synthetic diet where the sole source of N is enzyme-hydrolysed casein (EHC), which consists of small peptides (molecular weight < 5000 Da) and free AA. Digesta from the terminal ileum are collected, centrifuged and ultrafiltered (molecular weight exclusion limit of 10 000 Da). The precipitate from the centrifugation step and the retentate from the ultrafiltration step (>10 000 Da fraction of digesta) are considered to provide the estimate of the endogenous component of the digesta.
The objective of the experiment reported herein was to investigate the effects of PA and microbial phytase on the flow and composition of endogenous protein in the ileum of 28-d-old broiler chickens, using the peptide alimentation method. It was hypothesised that the presence of dietary PA would increase and phytase supplementation would reduce the flow of ileal endogenous AA in growing broiler chickens.
Materials and methods
Phytic acid and microbial phytase
The PA (myo-inositol hexaphosphate) was added in a purified form (PA, sodium salt; EC no. 238-242-6; Sigma-Aldrich Corporation, St Louis, MO, USA).
The phytase product (Phyzyme XP™) was derived from Escherichia coli and expressed in Schizosaccaromyces pombe (Danisco Animal Nutrition, Marlborough, UK). The enzyme is a 6-phytase (EC 3·1·3·26) that commences the dephosphorylation of PA at position 6 of myo-inositol. The product contained a minimum phytase activity of 5000 FTU/g and addition of 100 g enzyme/tonne provided a guaranteed minimum of 500 FTU/kg diet. One unit of phytase (FTU) is defined as the quantity of enzyme which releases 1 μmol of inorganic phosphorus/min from 0·15 mm-sodium phytate at pH 5·5 at 37°C.
Diets
The study was conducted as a 2 × 3 factorial arrangement of treatments to evaluate the effects of three concentrations of PA (8·5, 11·5 and 14·5 g/kg; equivalent to 2·4, 3·2 and 4·0 g/kg phytate P) and two concentrations of microbial phytase (0 and 500 FTU/kg diet) on the ileal endogenous AA flow in broiler chickens. In addition, a seventh treatment (without PA and microbial phytase) was included to obtain estimates of basal endogenous AA flow.
A basal diet with the 200 g/kg EHC (Table 1) as the sole source of N was formulated. Based on this, six experimental diets were developed. A casein-based diet was also developed (Table 1), which was similar to the EHC diet except that casein was used in place of EHC. Titanium oxide (3 g/kg) was included in the test diets as an inert marker for the calculation of AA flows. Microbial phytase, in powder form on a starch base, and PA were accurately weighed, added in place of dextrose (w/w basis) in the basal diet and mixed well in a vertical mixer.
Table 1 Composition (g/kg, as-fed basis) of the casein diet and the basal enzyme-hydrolysed casein (EHC) diet
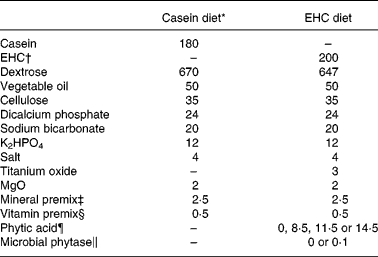
* Casein diet was fed to all birds for 3 d prior to the introduction of the experimental diets.
† New Zealand Pharmaceuticals Ltd, Palmerston North, New Zealand. The molecular weight distribution was determined using gel permeation chromatography. The sample was less than 5000 Da in size, with 99 % less than 3000 Da.
‡ Supplied per kg diet: Mn, 125 mg; Zn, 60 mg; Cu, 3 mg; Mo, 0·5 mg; Co, 0·3 mg; I, 1 mg; Fe, 25 mg; Se, 200 μg; choline chloride, 638 mg.
§ Supplied per kg diet: trans-retinol, 3·33 mg; cholecalciferol, 60 μg; dl-α-tocopheryl acetate, 60 mg; menadione, 4 mg; thiamine, 3·0 mg; riboflavin, 12 mg; calcium pantothenate, 12·8 mg; niacin, 35 mg; pyridoxine, 10 mg; folic acid, 5·2 mg; cyanocobalamin, 0·017 mg; biotin, 0·2 mg; antioxidant, 100 mg.
¶ Purified source (phytic acid, sodium salt); included in place of dextrose (w/w).
‖ Phyzyme XP™ 5000 (Danisco Animal Nutrition, Marlborough, UK); included in place of dextrose (w/w).
Birds
Male broiler (Ross 308) chicks were obtained at 1 d old from a commercial hatchery and raised in floor pens and fed a standard commercial diet. On day 14 post-hatch, the birds were individually weighed and 210 birds of similar body weight range were selected and assigned to forty-two cages of five birds each. The seven dietary treatments were then randomly assigned to six cages each. The cages were housed in an environmentally controlled room with 24 h fluorescent lighting. Room temperature was maintained at 24 ± 2°C during the assay.
Experimental procedures were approved by the Massey University Animal Ethics Committee and complied with the New Zealand Code of Practice for the Care and Use of Animals for Scientific Purposes.
Conduct of the trial
From days 14–21 post-hatch, the birds were first fed a mash diet, which was made by grinding commercial starter pellets in a hammer-mill to pass through a 3 mm screen. The casein diet (Table 1) was gradually introduced from days 21–24 post-hatch. The aim of using the casein diet was to enable the birds to adjust to the changeover to EHC-based experimental diets. The casein diet was withdrawn on day 24 and the experimental diets were introduced and offered for 2 d. The diets were offered ad libitum and water was available at all times.
Digesta collection and processing
On day 26, all birds were killed by an intravenous injection of sodium pentabarbitone and digesta from the distal one-half of the ileum were collected. Samples from birds within a cage were pooled, homogenised, frozen immediately after collection and lyophilised. The following procedure was used to separate the endogenous protein fractionReference Ravindran, Hew, Ravindran and Bryden10. The lyophilised samples were re-suspended in deionised water and acidified to pH 3·5 with 9 m-H2SO4. The samples were stored overnight at 4°C and then centrifuged at 1450 g for 45 min at 0°C. The supernatant was decanted off and retained. The precipitate was washed with 10 ml deionised water and centrifuged at 1450 g for 30 min at 0°C. The second supernatant was added to the first and the precipitate was stored at − 20°C. The combined supernatants were ultrafiltered using a Centriprep-10 ultrafiltering device (molecular weight cut-off filter, 10 000 Da; Amicon Inc., Beverly, MA, USA) according to the manufacturer's instructions. The precipitate from the centrifugation step was added to the retentate (>10 000 Da) from the ultrafiltration step, and the material was lyophilised. Diets and digesta were then ground to pass through a 0·5 mm sieve and stored in airtight containers at − 4°C for chemical analyses.
Chemical analyses
The diets and ultrafiltered ileal digesta samples were analysed for DM, N, AA and titanium as described later.
DM determinations were carried out according to Association of Official Analytical Chemists procedures11. Total N was determined by the Dumas methodReference Sweeney12 using a CNS-2000 carbon, nitrogen and sulphur analyser (LECO Corporation, St Joseph, MI, USA). AA were determined by hydrolysing the samples with HCl (containing phenol) for 24 h at 110 ± 2°C in glass tubes sealed under vacuum. AA were detected on a Waters ion exchange HPLC system, and the chromatograms were integrated using dedicated software (Maxima 820; Waters, Millipore, Milford, MA, USA) with the AA identified and quantified using a standard AA solution (Pierce, Rockford, IL, USA). Cyst(e)ine and methionine were analysed as cysteic acid and methionine sulphone by oxidation with performic acid for 16 h at 0°C and neutralisation with hydrobromic acid prior to hydrolysis. Cysteine was expressed as cystine. Tryptophan was not determined. Titanium content was measured on a UV spectrophotometer following the method of Short et al. Reference Short, Gorton, Wiseman and Boorman13. Phytase activity in the finished diets was determined according to the method of Engelen et al. Reference Engelen, van der Heeft, Ransdorp, Somers, Schaefer and van der Vat14 with some modifications. These modifications were associated with the extraction buffer, which was 0·25 m-acetate (pH 5·5) with 0·01 % Tween 20 and 1 mm-CaCl2, which has been optimised for this phytase product (Phyzyme XP™).
Calculations
The endogenous flow of N and individual AA at the terminal ileum was calculated as mg lost per ingestion of kg feed DM, using the formulaReference Moughan, Schuttert and Leenaars15: Endogenous N or AA flow (mg/kg DM intake) = N or AA concentration in ileal digesta (mg/kg) × (Diet titanium (mg/kg)/Ileal digesta titanium (mg/kg)).
The AA profile of endogenous protein (N × 6·25) was calculated by expressing each AA as a percentage of endogenous crude protein.
Statistical analysis
Two-way ANOVA was employed to determine the main effects (PA and phytase) and their interaction by using the GLM procedure of SAS version 6.12 (SAS Institute, Cary, NC, USA) using cage as the experimental unit. The data from the EHC diet without added PA were compared with those containing 8·5 g/kg PA using a completely randomised design ANOVA. Differences were considered significant at P < 0·05, although probability values up to P ≤ 0·1 are shown in the text if the data suggest a trend. When a significant F-test was detected, treatment means were separated using the least significant difference test.
Results
The determined crude protein and AA composition of the EHC diet with no added PA are shown in Table 2. The determined phytase activity in the finished diets is presented in Table 3. The average recovery was 92 %, which is acceptable given the variance in sampling, mixing and assay sensitivity.
Table 2 Determined crude protein and amino acid concentrations of the basal enzyme-hydrolysed casein diet without added phytic acid
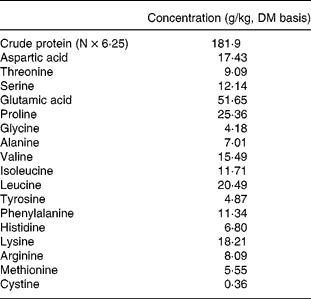
Table 3 Recovery of phytase activity in the experimental diets

FTU, phytase unit.
The influence of graded concentrations of PA and phytase supplementation in the ileal endogenous flows of N and AA is shown in Table 4. The effects of dietary PA concentration on ileal endogenous flow were significant (P ≤ 0·05–0·001) for N and some AA, with the flow increasing with increasing PA additions. The flow of proline, alanine, valine, tyrosine, phenylalanine, histidine, lysine and arginine were not influenced (P>0·1) by phytate concentration. Phytase supplementation lowered (P < 0·1–0·001) the ileal endogenous flow of most of the AA. The exceptions were the flow of alanine, tyrosine and phenylalanine, which were unaffected by added phytase. Responses to phytase supplementation in the flow of aspartic acid, phenylalanine, histidine, arginine, cystine and methionine, however, were influenced by dietary PA concentration, as indicated by PA × phytase interactions (P < 0·1–0·05). Supplemental phytase had no effect on the flow of histidine, arginine, cystine and methionine at the 8·5 g/kg PA concentration, but lowered the flow at 11·5 and 14·5 g/kg PA concentrations. The flow of phenylalanine was not affected by supplemental phytase at 8·5 and 11·5 g/kg PA concentrations, but lowered at 14·5 g/kg PA concentration. The flow of aspartic acid was lowered by supplemental phytase at all PA concentrations, but the reductions tended to be greater at the 8·5 g/kg PA concentration.
Table 4 Ileal endogenous flows (mg/kg DM intake) of nitrogen and amino acids (AA) in 28-d-old broiler chickens as influenced by dietary levels of phytate and microbial phytase (Mean values for six replicates of five birds each with their pooled standard errors)

FTU, phytase unit; PA, phytic acid.
* Differences in the flow of N and most AA between birds fed diets with 0 and 8·5 g/kg phytic acid were significant (P < 0·10–0·001), except for the flow of alanine and phenylalanine.
† Data involving 8·5, 11·5 and 14·5 g/kg phytic acid, without or with microbial phytase, were analysed as a 3 × 2 factorial arrangement of treatments.
The comparison of ileal endogenous flow of N and AA in birds fed the EHC diet without PA or with 8·5 g/kg PA is also presented in Table 4. The endogenous flow of N and most of the AA were greater (P < 0·05–0·001) in birds fed the diet with PA compared to those fed the diet with no added PA. The flow of histidine, cystine and methionine tended (P < 0·1) to be greater in birds fed the diet with PA. No differences (P>0·1) were observed for the flow of alanine and phenylalanine. The ileal flow of N and total AA in birds fed the EHC diet with 8·5 g/kg PA were 1·4 times as much as those determined for that with no added PA. The increments in the flow of individual AA in birds fed the diet with PA ranged from 11 % (methionine) to 97 % (threonine).
The influence of dietary treatments on the AA profile of endogenous protein, expressed as g/100 g crude protein, is summarised in Table 5. The concentrations of aspartic acid, threonine, serine and tyrosine were increased (P < 0·05–0·001) and those of glutamic acid, alanine and phenylalanine were lowered (P < 0·1–0·05) in the endogenous protein of birds fed the EHC diet with 8·5 g/kg PA compared to those fed the diet with no added PA. The concentrations of other AA in endogenous protein were unaffected (P>0·1).
Table 5 Amino acid (AA) composition of endogenous protein (g/100 g crude protein) in 28-d-old broiler chickens as influenced by dietary levels of phytate and microbial phytase (Mean values for six replicates of five birds each with their pooled standard errors)

FTU, phytase unit; PA, phytic acid.
* Differences in the flow of N and most AA between birds fed diets with 0 and 8·5 g/kg phytic acid were significant (P < 0·05) for the flow of aspartic acid, threonine, serine, tyrosine and phenylalanine. Tendency (P < 0·10) for significance was found for glutamic acid and alanine.
† Data involving 8·5, 11·5 and 14·5 g/kg phytic acid, without or with microbial phytase, were analysed as a 3 × 2 factorial arrangement of treatments.
Increasing dietary concentrations of PA influenced (P < 0·05–0·001) concentrations of aspartic acid, serine, proline, glycine, valine, leucine and histidine in endogenous protein (Table 5). The concentrations of all AA, except proline, isoleucine, leucine, tyrosine, histidine, lysine, arginine and methionine, in the endogenous protein were lowered (P < 0·1–0·001) by phytase supplementation. Significant PA × phytase interactions (P < 0·1–0·01) were noted for aspartic acid, proline, alanine, phenylalanine, histidine, lysine, arginine, cystine and methionine, indicating that the responses in the concentrations of these AA in endogenous protein with supplemental phytase varied at different dietary PA concentrations.
Discussion
The present results, along with previous reportsReference Cowieson, Acamovic and Bedford5, Reference Onyango, Asem, Sands and Adeola16, confirm that PA is an anti-nutrient capable of stimulating an increase in the flow of endogenous material in the small intestine of broiler chickens and that the capacity of PA as an anti-nutrient is reduced in the presence of microbial phytase. It is noteworthy that the composition of basal endogenous protein from the current study is very similar to that of a previous study, which employed the precision feeding technique involving fastingReference Cowieson, Acamovic and Bedford5. In both studies, glutamic acid, aspartic acid and leucine were the most significant contributors to endogenous protein composition. In fact, when the AA composition of endogenous protein is expressed as a percentage of the total, the data from the two studies were highly correlated (data not shown) with a R 2 of 0·83. This is contrary to the finding of Smirnov et al. Reference Smirnov, Sklan and Uni17 who reported significant alterations in mucin dynamics in the small intestine of chickens during starvation. The current data suggest that the precision feeding method may not be as compromised as previously thoughtReference Smirnov, Sklan and Uni17 as a bioassay for monitoring basal endogenous flow. Further, the fact that the composition of basal endogenous protein is similar in two studies based on different methods (precision feeding v. protein alimentation) increases confidence in the results.
In the current study, there was not only a significant increase in total endogenous AA flow with increasing PA ingestion but also significant changes in the composition of the endogenous protein (Table 5). Most notably the flows of aspartic acid, serine, threonine and tyrosine were increased as the dietary concentration of PA was increased. This observation suggests that PA does not simply increase total endogenous protein flow but selectively increases the flow of some endogenous protein sources more than others, altering the AA profile of endogenous protein. It has been previously demonstrated that PA increases the secretion of mucoprotein in the intestine of growing broilersReference Cowieson, Acamovic and Bedford5, Reference Cowieson, Acamovic and Bedford18. Because mucins are particularly rich in threonine, serine, proline and cysteineReference Forstner, Forstner and Johnson19, it can be speculated that if PA increases the secretion of mucin relative to other endogenous protein sources that these AA would be those most obviously increased. Data from the present study would strongly support this contention with the exception of cysteine. However, the core peptides of mucin consist of two main domains, a major domain that is rich in serine, threonine and proline, and a minor domain that is rich in cysteineReference Forstner, Forstner and Johnson19. It is possible that the ingestion of PA increases the secretion of mucins with a relatively higher concentration of major domain mucoprotein. The increased flow of mucoprotein at the terminal ileum has significant implications both for the net energy value of the diet and also for the protection of the gastro-intestinal tract against pathogens and other dietary nutrients. It is possible therefore that diets that contain a high concentration of PA, in the absence of phytase, would lower net energy contribution and increase the susceptibility of birds to enteric diseases.
Other sources of endogenous loss include pancreatic and brush-border enzymes. The AA composition of endogenous enzymes has received some attention in the literature on swine nutrition but scant data exist for poultry. Trypsin contains a relatively high concentration of alanine, glycine and serine20. Pepsin is particularly rich in leucine, glycine and aspartic acid20. Lipase has a high concentration of glycine, aspartic acid and leucine20. The AA composition of endogenous amylase is primarily glycine-, alanine-, serine- and valine-based whereas for endogenous maltase serine, valine and leucine predominate20. Thus, for endogenous enzymes the AA of most significance are serine, leucine and glycine. Interestingly endogenous enzymes are essentially devoid of methionine, an AA that is not particularly affected by either phytate or phytase (Table 5). These data would tend to suggest that an increase in the secretion of endogenous enzymes, particularly pepsin, is possible with the ingestion of PA. The increased secretion of pepsin may be a consequence of de novo formation of protein–phytate complexes in the gastric phase of digestion, leading to additional pepsin and likely also HCl secretion in an attempt to solubilise protein. Compositional changes in the endogenous protein also suggest that the starch-degrading enzymes are also affected by the presence of phytate in the gut lumen. This may be mediated via complexing of calcium, a necessary co-factor for amylase, leading to an increased pancreatic amylase secretion and changes in the secretion of maltase and isomaltase at the brush border. It has been found previously that poultry pancreatic amylase is irreversibly inactivated following calcium removal whereas for other amylase sources the deleterious effects can be reversed by the addition of calciumReference Buonocore, Deponte, Gramenzi, Petrucci, Poerio and Salino21. Indeed, previous reports have found that PA reduces the digestion of starches by amylase, both in vitro and in vivo Reference Knuckles and Betschart22, Reference Cowieson, Acamovic and Bedford23. Data from the current experiment would tend to support the contention that alterations in amylase and maltase secretion may be involved in the inimical effects of PA on starch digestion, with secondary effects on energy and AA requirements.
Phytase has been shown to improve the retention of P, Ca, some trace minerals, energy and also AAReference Selle and Ravindran2, Reference Cowieson, Acamovic and Bedford24, Reference Ravindran, Morel, Partridge, Hruby and Sands25, particularly in diets with available P, Ca and energy contents below the requirements. The mode of action of phytase on the retention of P, Ca and other minerals is relatively well elucidated, being linked to the release of orthophosphate from the inositol nucleus following hydrolysis of the ester bonds in phytate and a decrease in the chelating capacity of the lower molecular weight inositol phosphate esters. However, data to support the effect of phytase on the retention of AA have been equivocal and confidence in AA matrix values for currently available microbial phytase products in the market is weak. The data presented herein present a convincing mode of action and this may assist in the interpretation of available data on AA digestibility responses following phytase supplementation. Selle et al. Reference Selle, Ravindran, Bryden and Scott26 present a comprehensive review of phytase and AA digestibility in poultry, and offer three potential mechanisms by which phytase can improve the AA ‘value’ of a diet. Firstly, the presence of protein–phytate complexes in feedstuffs, reducing solubility (and digestibility) of dietary protein; secondly, the de novo formation of binary and ternary complexes between protein, minerals and phytate in the gastro-intestinal tract; and finally the inhibition of proteolytic enzymes or their cofactors by phytate. A final mechanism, supported by the data presented herein, is that phytase elicits its positive effects on AA digestibility, in part, via a reduction in endogenous AA flow. The extent to which each of the three proposed mechanisms contributes to the approximately 3–6 % improvement in apparent ileal AA digestibility in poultry is clearly open to debate. However, the data presented herein suggest that improvements in the ileal digestibility of alanine, phenylalanine and tyrosine that have been reportedReference Selle, Ravindran, Bryden and Scott26 are not mediated via a reduction in endogenous AA flow but must be via one of the other proposed mechanisms. The relatively weak effect of phytase on methionineReference Selle, Ravindran, Bryden and Scott26 may be due to the fact that methionine is an AA that is not present in high concentrations in endogenous secretions and so the endogenous ‘cost’ is not as high as for other AA. The digestibility of AA such as threonine, serine, aspartic acid, glycine, leucine, lysine and cysteine which are improved substantially by phytase may have a large portion of the benefit explained by a reduced endogenous flow.
It must be also noted that the flow of endogenous protein represents a balance between secretion and re-absorption. Both these aspects can be influenced by a number of animal and dietary factors, especially anti-nutritive factorsReference Nyachoti, de Lange, McBride and Schulze4. Thus the increased endogenous AA flow with the ingestion of PA and the reduced flow with phytase addition may be due not only to changes in the secretion of mucin and endogenous enzymes, but also reflect changes in the efficiency of re-absorption or both.
In accord with previous workReference Cowieson, Acamovic and Bedford5, the flow of endogenous N was significantly increased with the ingestion of PA and reduced by supplemental phytase. The majority of this endogenous N is associated with endogenous AA but a proportion is of non-protein origin. Indeed, the 11 520 mg basal endogenous AA flow (Table 4) would contribute only 1843 mg N (assuming an average AA N content of 160 g/kg) compared with the 2051 mg N lost by the birds. This discrepancy may be from AA that were not measured in the current experiment such as tryptophan, but more likely the difference is associated with an increase in the flow of non-protein N. Interestingly, AA N as a proportion of total N in the ileal flow increased with PA ingestion from around 89 % in the basal endogenous flow to over 95 % with the ingestion of PA. This may be of little significance but it is notable and suggests either changes in the flow of AA which were not determined or changes in non-protein N flow.
The EHC method was used in the current study for the measurement of ileal endogenous AA flow. A number of assumptions are made when this method is usedReference Nyachoti, de Lange, McBride and Schulze4, Reference Moughan, Souffrant and Hodgkinson27. Most of these assumptions have been tested and appear to be valid. A potential limitation is that there may be some underestimation of endogenous AA flow due to a loss of low molecular weight endogenous peptides and AA in the ultrafiltrateReference Moughan, Souffrant and Hodgkinson27. In the current study, however, the same diet (200 g EHC/kg diet) served as the basal formula for all treatments. Thus the effect of any underestimation in endogenous AA flow is inherent to this diet and will not influence the comparisons between the treatments employed.
In conclusion, the results of the current study demonstrate that PA is a potent anti-nutrient and represents a significant nutritional obstacle in poultry diets. The flow of endogenous N and AA is markedly increased with the ingestion of PA but the presence of microbial phytase is effective in reducing these adverse effects on endogenous investment. Thus, the effects of phytase on apparent ileal AA digestibility that have been reported may be explained, in part, via a reduction in endogenous flow. The information presented herein offers a convincing mechanistic argument for the improvements in AA digestibility in poultry with phytase supplementation.
Acknowledgements
Dr Mai Faurschou Isaksen, Danisco Innovation, Denmark, is thanked for assistance in obtaining the AA profiles of endogenous enzymes and for constructive discussions during the preparation of the paper.









