Hypertension, defined as systolic blood pressure (SBP) >140 mmHg and/or diastolic blood pressure (DBP) >90 mmHg, is a major risk factor for CVD. In fact, hypertension is the leading risk factor contributing to global mortality and affects 40 % of adults worldwide. For this reason, the World Health Organization( 1 ) has identified hypertension as a global public crisis. Early detection and treatment are vital to reduce cardiovascular risk and to prevent cardiovascular events( Reference Lorente-Cebrian, Costa and Navas-Carretero 2 ). Therefore, it is important to identify effective strategies to reduce the incidence and prevalence of hypertension, and in this respect, an optimal dietary intake is a proven preventive and supportive strategy.
Evidence suggests that EPA (20 : 5n-3) and DHA (22 : 6n-3) can lower BP at daily intakes of more than 2 g, with hypertensive individuals being more responsive( Reference Geleijnse, Giltay and Grobbee 3 – Reference Miller, Van Elswyk and Alexander 5 ). As EPA and DHA, α-linolenic acid (ALA) is a fatty acid (FA) that belongs to the n-3 PUFA family. It is an essential FA mainly derived from plants and found in, for example, nuts, leafy vegetables and plant seed oils such as rapeseed, soyabean and flaxseed oils. Like EPA and DHA, specific dietary guidelines have been formulated for ALA (0·5–1·0 % energy (En%) or about 1–2 g), which are often not met( Reference Sioen, van Lieshout and Eilander 6 ). Although limited, ALA can be converted into EPA and to an even smaller extent into DHA( Reference Goyens, Spilker and Zock 7 ). Like n-3 long-chain PUFA, ALA may lower BP( Reference Baxheinrich, Stratmann and Lee-Barkey 8 – Reference Takeuchi, Sakurai and Noda 11 ). However, several other studies found no effects of ALA on BP( Reference Barcelo-Coblijn, Murphy and Othman 12 – Reference Wilkinson, Leach and Ah-Sing 15 ). Noteworthy, none of these trials were performed in subjects with increased BP. In fact, Estruch et al. ( Reference Estruch, Martinez-Gonzalez and Corella 16 ) have reported that hypertensive subjects showed significantly larger reductions in SBP than normotensive subjects when given a Mediterranean diet high in ALA. In view of these conflicting findings, we conducted a 12-week, double-blind, randomised parallel, placebo-controlled study to investigate the effects of ALA consumption (4·7 g/d, approximately 2 % of energy intake) on 24-h ambulatory BP (ABP) in a population with high-normal BP or mild hypertension.
Methods
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving humans were approved by the medical ethical committee of Maastricht University Medical Centre (METC 13-3-064) and registered at ClinicalTrials.gov as NCT02243969. Written informed consent was obtained from all subjects before entering the study.
Study population
Healthy, overweight or obese subjects with a BMI between 25 and 35 kg/m2 and aged between 40 and 70 years were recruited in and near the vicinity of Maastricht by means of posters in the university and hospital buildings and advertisements in local newspapers. In addition, subjects who had participated in our earlier studies were approached. Potential subjects were invited for two screening visits for the measurement of office BP, height and weight. In addition, a fasting blood sample was taken for analysis of plasma glucose and serum lipid concentrations. Furthermore, subjects were asked to complete a medical and general questionnaire. Eligible for participation were non-smoking men and women with a high-normal BP defined as SBP between 130 and 139 mmHg and/or DBP between 85 and 89 mmHg or stage I hypertension defined as SBP between 140 and 159 mmHg and/or DBP between 90 and 99 mmHg during both screening visits, mean serum total cholesterol:HDL-cholesterol ratio <8, mean serum TAG <4·5 mmol/l and mean plasma glucose <7·0 mmol/l. Subjects with active CVD or severe medical conditions that might interfere with the study outcomes, use of non-steroidal anti-inflammatory drugs, anti-hypertensive, anti-coagulant medication or a diet/medication known to affect serum lipid or glucose metabolism were excluded. Other exclusion criteria were an unstable body weight (weight gain or loss >2 kg in the past 3 months); indication for treatment with medication according to the standard for cardiovascular risk management of the Dutch general practitioners community (Nederlands Huisartsen Genootschap; NHG); drug or alcohol abuse; intense sporting activities >10 h/week; use of nutritional supplements; women expecting changes in the use of oral contraceptives during the study period or lactating, pregnant or intend to become pregnant during study. Finally, subjects had to be willing to give up being a blood donor from 8 weeks before the start of the study and during the study.
Study design
The study had a randomised, double-blind, placebo-controlled parallel design and consisted of a 2-week run-in period followed by a 12-week intervention period. During the entire 14 weeks, subjects were asked to consume daily 5 g of oil at breakfast or lunch and 5 g at dinner. The oils were provided in vials containing 5 g of oil, which were letter coded to blind the subjects and the investigators. During the run-in period, all subjects consumed palm supra olein oil. For the intervention period, they were randomly allocated to either the control or the intervention group, stratified for sex. Subjects in both the groups switched to another treatment oil to maintain blinding. The intervention group received refined cold-pressed flaxseed oil, providing approximately 4·7 g ALA and the control group received high-oleic sunflower oil (Table 1). Subjects were provided with a number of oil vials sufficient for the days until the next visit plus 2 d spare. They were asked to return all unused vials that were counted as a measure of compliance. Although the subjects were free to take the oils according to their own preferences, advice was given to mix the oil with liquid foods such as yogurt, sauces and salad dressing or to use it with bread. It was not allowed to use the oils for baking or frying.
Table 1 Fatty acid (FA) composition of the experimental oils

During the 2-week run-in period, subjects visited the research unit at days 0, 11 and 14. The measurements at the end of the run-in period (days 11 and 14) served as baseline measurements. During the 12-week intervention period, subjects visited the university after 6 weeks (day 56) and twice in week 12 (days 95 and 98). At each visit, body weight and office BP were measured, and a fasting blood sample was obtained. In all, 24-h ABP was monitored at the end of the run-in period between days 11 and 14 (baseline) and at the end of the intervention period between days 95 and 98. Food intake over the previous month was assessed at days 14 and 98 by a validated FFQ( Reference Plat and Mensink 17 ). Questionnaires were checked by a dietitian, and energy and nutrient intake were calculated using the Dutch food composition table( 18 ). Subjects were asked to keep their habitual diet, level of physical exercise and alcohol intake throughout the study and to refrain from the consumption of vitamin supplements, capsules providing n-3 long-chain PUFA, and products rich in plant stanol or sterol esters three weeks before the start and during the study. Any protocol deviations, signs of illnesses and use of medication or alcohol consumption were recorded in a study diary and was discussed and inspected at each visit. All measurements were performed at the Metabolic Research Unit Maastricht of Maastricht University.
Measurements
Office BP and heart rate (HR) were determined according to the American Heart Association recommendations for BP measurements in humans( Reference Pickering, Hall and Appel 19 ). Measurements were performed in a seated position after at least 5 min of rest on the left upper arm in fourfold with 1-min intervals using a calibrated Omron device (Omron M7; CEMEX Medische Techniek BV). The first measurement was discarded, and three subsequent measurements were averaged. Mean arterial pressure (MAP) was calculated as 1/3×SBP+2/3×DBP, while pulse pressure (PP) was calculated by subtracting the DBP from the SBP.
The 24-h ABP was monitored using an automated BP device (Mobil-O-Graph NG; APC Cardiovascular). BP and HR were measured at 15-min intervals during the day (07.00–23.00 hours) and at 30-min intervals during the night (23.00–07.00 hours). Subjects were asked to maintain their normal daily activities during the recording period and to avoid intense exercise. By pressing a button on the device when going to sleep and waking up, subjects indicated the start of the night and day period. In all, 24-h ABP as well as night-time BP were calculated, which was defined as the average of BP from the time the subjects went to bed until the time they woke up. Daytime BP was defined as the average of BP measurements recorded during the rest of the day. The fall in BP during the night, called night-time dipping, was defined as the difference between average daytime and average night-time BP expressed as a percentage of the daytime value.
At each visit to the research unit, venous blood was sampled after an overnight fast. On days preceding blood drawings, subjects were asked to avoid the intake of alcohol and not to take part in any strenuous activity. The same person performed each venipuncture at approximately the same time of the day. For the analysis of the FA profile of plasma phospholipids, blood was sampled on days 14 and 95 in EDTA-containing tubes, kept on ice and centrifuged at 1300 g for 10 min at 4°C without break to obtain plasma. Aliquots were capped under an N flow and stored at –80°C until analysis. After the study, total lipids were first extracted from plasma by means of a modified procedure of the Folch method as described earlier( Reference Goyens, Spilker and Zock 7 , Reference Goyens, Spilker and Zock 20 ). Next, phospholipids were isolated from the total lipid extract on an Extract-Clean NH2-aminopropylsilyl column. FA methyl esters were prepared as described( Reference Goyens, Spilker and Zock 7 , Reference Goyens, Spilker and Zock 20 ) and separated and quantified using triple quadrupole GC–MS (Agilent 7000B GC–MS/MS EI system).
Statistical analysis
Results are presented as means and standard deviations. Where available, individual values of measurements from days 11 and 14 (end of run-in) and of days 95 and 98 (end of intervention) were averaged before statistical analysis. When twenty-five subjects in each group completed the study, the statistical power was more than 80 % to detect a true difference of 3·5 mmHg( Reference Takeuchi, Sakurai and Noda 11 ) in mean 24-h ambulatory MAP between the treatments, based on a standard deviation of 8 mmHg, a two-sided α of 0·05 and a correlation coefficient of 0·80 between measurements obtained at the end of the run-in and experimental periods( Reference Joris, Plat and Bakker 21 ). Data were analysed using a per-protocol approach. Baseline values of the two groups were compared using an unpaired Student’s t test. Differences in responses between the flaxseed oil and control groups were evaluated by a one-way ANCOVA with baseline values of the outcome variable as covariate. Differences were considered statistically significant at a two-sided significance level of P<0·05. Statistical analyses were performed using SPSS 22.0 for Mac OS X (SPSS Inc.).
Results
Subject characteristics and compliance
Fig. 1 shows the flow of subjects through the study. A total of sixty-two subjects were randomly assigned to either the flaxseed oil group or the control group. In all, one male assigned to the flaxseed oil group dropped out because of a change in medication use. In all, two men assigned to the control group dropped out because of an unexpected change in work and an unplanned holiday. Data from fifty-nine subjects were available for analysis. Inspection of the diaries did not suggest any protocol deviations that may have affected the results. The screening characteristics of these subjects are shown in online Supplementary Table S1. Screening and baseline characteristics of the subjects in the control group were comparable to those in the flaxseed oil group (P>0·05 for all variables). Office BP and 24-h ABP were within the range of high-normal BP and stage I hypertension. Compliance was excellent as evidenced from the counts of returned vials, which ranged between 82 and 106 % and was on average 96 %. Apart from intakes of the FA under study, the composition of the diets was similar in both the groups (Table 2). As expected, the flaxseed oil group had a significantly higher intake of total PUFA and ALA, while intake of MUFA, mainly oleic acid, was higher in the control oil group. Body weights did not significantly change during the study and were 88·1 (sd 9·7) kg at the start and 88·5 (sd 9·8) kg at the end for the control group. For the flaxseed oil group, these values were, respectively, 85·3 (sd 10·5) and 86·1 (sd 10·9) kg.
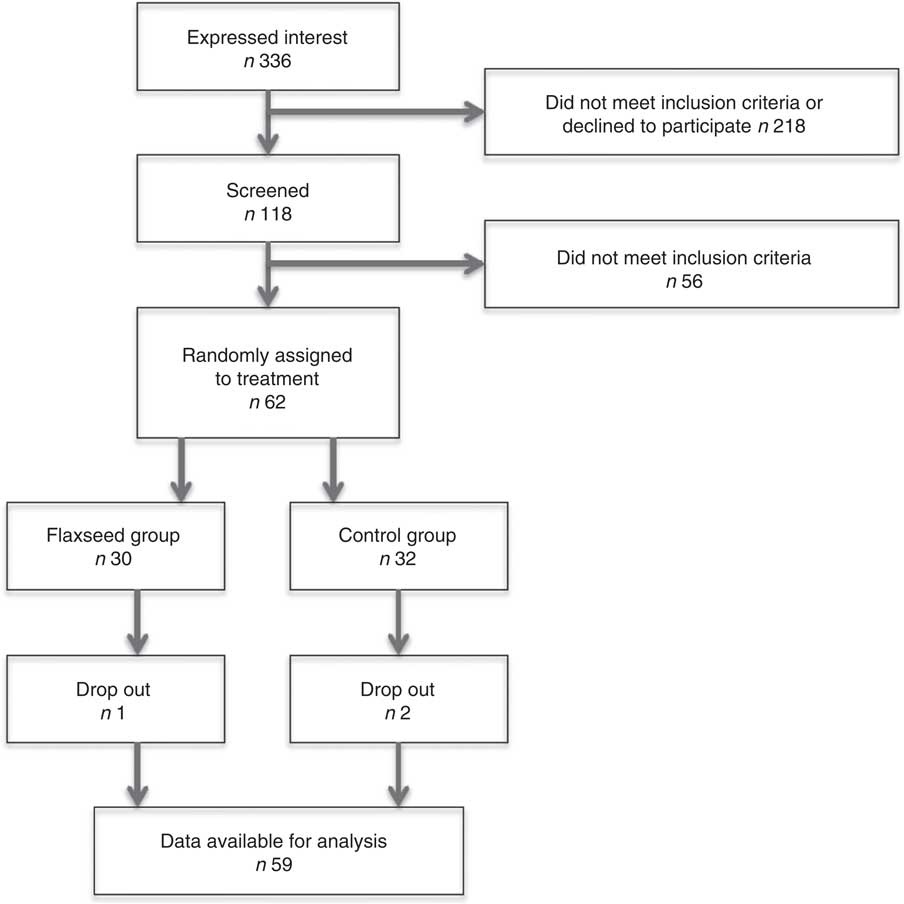
Fig. 1 Subject flow chart of progress through the phases of the study.
Table 2 Energy and nutrient intake at baseline and after 12 weeks of supplementation with 10 g/d flaxseed oil or high-oleic sunflower oil (HOSF) (Mean values and standard deviations; mean differences and 95 % confidence intervals)

En%, energy percentage.
* Mean difference in change between the flaxseed and control groups with 95 % CI obtained from a one-way ANCOVA with baseline value as covariate.
Blood pressure
When compared with the control group, average 24-h ambulatory MAP, SBP, DBP, PP and HR were not significantly affected in the flaxseed oil group (Table 3). Online Supplementary Fig. S1 shows for both the groups the mean hourly ambulatory SBP, DBP and HR as measured for 24 h at baseline and at the end of the intervention period. Separate analyses of daytime and night-time measures of MAP, SBP, DBP, PP and HR did not show differences between treatments. Also, no statistically significant effects were found on night-time dipping of SBP and DBP and on office measurements of MAP, SBP, DBP and HR (Table 3).
Table 3 Ambulatory and office blood pressure measurements at baseline and after 12 weeks of supplementation with 10 g/d flaxseed oil or high-oleic sunflower oil (HOSF) (Mean values and standard deviations; mean differences and 95 % confidence intervals)

SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; HR, heart rate; bpm, beats per min.
* Mean difference in change between the flaxseed and control groups with 95 % CI obtained from a one-way ANCOVA with baseline value as covariate.
Plasma phospholipid fatty acid composition
When compared with the high-oleic control oil, intake of flaxseed oil significantly increased the percentage of total PUFA and total n-3 PUFA in plasma phospholipids (Table 4). More specifically, ALA increased about 2·5-fold by 0·3 % point (P<0·01). Also, EPA and DPA significantly increased with 0·6 % point (P<0·01) and 0·2 % point (P<0·01), respectively. The percentage of DHA and total n-6 PUFA did not change significantly, while the percentage of total MUFA, mainly oleic acid, significantly decreased in the flaxseed oil group when compared with the control group. Also, the percentage of total SFA significantly increased and total trans-FA significantly decreased in the flaxseed oil group.
Table 4 Fatty acid composition of plasma phospholipids at baseline and after 12 weeks of supplementation with 10 g/d flaxseed oil (n 28) or high-oleic sunflower oil (n 28)* (Mean values and standard deviations; mean differences and 95 % confidence intervals)

* Values are expressed as % (w/w) of total fatty acids identified. Only SFA ≥C14 are included.
† Mean difference in change between the flaxseed and control groups with 95 % CI obtained from a one-way ANCOVA with baseline value as covariate.
Discussion
In this well-controlled study with untreated (pre-)hypertensive subjects, increasing daily ALA intake to 2·8 En% for 12 weeks, which is about 3–5 times the recommended intake( Reference Sioen, van Lieshout and Eilander 6 ), had no effect on BP when compared with the high oleic acid control. Compliance was excellent as indicated by counts of returned vials with the supplemental oils and observed changes in plasma phospholipid FA composition. Other studies that have examined effects of increased ALA consumption on BP have yielded conflicting results. Effects on BP were mainly found in subjects with high-normal BP or hypertension( Reference Baxheinrich, Stratmann and Lee-Barkey 8 , Reference Takeuchi, Sakurai and Noda 11 , Reference Rodriguez-Leyva, Weighell and Edel 22 ) but not in non-hypertensive subjects( Reference Finnegan, Minihane and Leigh-Firbank 13 – Reference Wilkinson, Leach and Ah-Sing 15 , Reference Bemelmans, Broer and Feskens 23 ). Therefore, we specifically focused on untreated high-normal and stage I hypertensive subjects. However, our results do not indicate that the discrepant results between the earlier studies can be explained by differences in baseline BP level. Also, the use of anti-hypertensive medication in other studies, which was one of the exclusion criteria in our study, is not likely to be a confounding factor, as decreases in BP with increased ALA have been observed both in studies on subjects with( Reference Baxheinrich, Stratmann and Lee-Barkey 8 , Reference Rodriguez-Leyva, Weighell and Edel 22 , Reference Vuksan, Whitham and Sievenpiper 24 ) and without( Reference Sioen, Hacquebard and Hick 10 , Reference Takeuchi, Sakurai and Noda 11 , Reference Paschos, Magkos and Panagiotakos 25 ) anti-hypertensive medication. Furthermore, dose and duration cannot be an explanation for the lack of effect on BP in our study. In previous 12-week studies on ALA, reductions in BP have been found with lower daily intakes of ALA – 2·6 g( Reference Takeuchi, Sakurai and Noda 11 ) and 3·2 g/d( Reference Sioen, Hacquebard and Hick 10 ) – when compared with the 4·7 g used in our study. In other experiments, the degree of blinding could have influenced the results. Of the positive studies, only the study of Takeuchi et al. ( Reference Takeuchi, Sakurai and Noda 11 ) was double blinded, while other studies were single blinded( Reference Sioen, Hacquebard and Hick 10 , Reference Vuksan, Whitham and Sievenpiper 24 ) or not blinded at all( Reference Baxheinrich, Stratmann and Lee-Barkey 8 , Reference Paschos, Magkos and Panagiotakos 25 ). Of the studies that did not show an effect on BP, the majority was single-( Reference Wilkinson, Leach and Ah-Sing 15 ) or double blinded( Reference Finnegan, Minihane and Leigh-Firbank 13 , Reference Kestin, Clifton and Belling 14 , Reference Bemelmans, Broer and Feskens 23 ); only one study was not blinded( Reference Barcelo-Coblijn, Murphy and Othman 12 ). Also, it can be speculated that the FA used to replace ALA intake may have influenced the results. Most of the earlier studies used linoleic acid as control for ALA, while we used oleic acid. However, there are no indications that oleic acid and linoleic acid have differential effects on BP( Reference Mensink, Stolwijk and Katan 26 ). One other trial reported effects of ALA on BP using oleic acid as control, although in that study hypo-energetic diets were provided( Reference Baxheinrich, Stratmann and Lee-Barkey 8 ). Another possible explanation for discrepant findings between studies could be the source of ALA used. In our study, we used ALA from purified cold-pressed flaxseed oil. Other studies used different sources of ALA, such as mixtures of refined vegetable oils( Reference Baxheinrich, Stratmann and Lee-Barkey 8 , Reference Takeuchi, Sakurai and Noda 11 , Reference Wilkinson, Leach and Ah-Sing 15 ), a specific type of whole grain (Salba)( Reference Vuksan, Whitham and Sievenpiper 24 ) or milled flaxseed( Reference Rodriguez-Leyva, Weighell and Edel 22 ). Less purified sources of ALA may contain other bioactive ingredients, such as lignans, peptides, minerals and fibres, that could have cardiometabolic effects( Reference Vuksan, Whitham and Sievenpiper 24 , Reference Bassett, Rodriguez-Leyva and Pierce 27 ). However, the effects of these components reported on BP are also not consistent, and sources of ALA in other trials were heterogeneous, and this explanation therefore remains speculative. Taken together, there is no obvious reason that can explain why in our study, unlike in some earlier studies, ALA supplementation did not affect BP. As the design and execution of our study was more rigourous than most of the earlier studies, a conceivable explanation is that ALA intake has no substantial effect on BP and that earlier positive findings were due to chance or potential confounding factors.
A strength of this study is that 24-h ABP measurements were used, which may better predict CVD than office BP( Reference Dolan, Stanton and Thijs 28 ). It also allowed us to examine the effects on daytime or night-time BP separately, which were also not significantly affected by flaxseed oil intake. Further, effects on dipping could be calculated, which is the decrease in SBP and DBP during night-time. Subjects in whom the BP reduction during sleep is less pronounced have an increased risk of cardiovascular events and mortality( Reference Yano and Kario 29 ). The observed values in our study could be classified as a normal dipping pattern( Reference Yano and Kario 29 ) and remained unaffected during the study.
Analysis of the FA composition of plasma phospholipids not only showed excellent dietary compliance but also showed that the intake of ALA increased EPA and DPA plasma phospholipid levels. This agrees well with results of other studies( Reference Goyens, Spilker and Zock 20 ).
In conclusion, we found that higher dietary intakes of ALA, about 3–5 times the recommended daily amounts, for 12 weeks does not significantly affect 24-h ABP or office BP in subjects with (pre-)hypertension.
Acknowledgements
The authors thank all volunteers for participating in the study, and Maurice Konings and Daisy Luiten for technical support.
This work was partially funded by Unilever Research and Development Vlaardingen, The Netherlands. Unilever marketed spreads rich in ALA before divesting its spreads business, which is since 2 July 2018 operating under the name of Upfield.
D. J. P. and R. P. M. carried out the study and performed the statistical analyses. All authors designed the study, interpreted the data and wrote the manuscript.
D. J. P. and R. P. M. report no conflict of interest. P. L. Z. is and D. F. was employed by Unilever R&D Vlaardingen, Vlaardingen, The Netherlands.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518003094










