The importance of good nutrition in maintaining optimal health and delaying disease progression among HIV-infected patients has been documented in the literature( Reference Fields-Gardner and Campa 1 , Reference Polsky, Kotler and Steinhart 2 ). At the beginning of the HIV epidemic, wasting was one of the main nutritionally related concerns; however, rates of obesity are now more common than the occurrence of wasting( Reference Amorosa, Synnestvedt and Gross 3 – Reference Mankal and Kotler 5 ), particularly in patients on antiretroviral treatment (ART)( Reference Crum-Cianflone, Roediger and Eberly 6 ).
An obesity paradox has been proposed in many conditions including HIV infection, where those who are obese may have a survival advantage or improved disease outcomes( Reference Kalantar-Zadeh, Horwich and Oreopoulos 7 ). The protective effect of obesity has been hypothesised to be related to extra available energy in the form of fat for use in times of crises, thus sparing protein use( Reference Carlson, Snead and Campbell 8 ), and also to help preserve the immune system response( Reference Shor-Posner, Campa and Zhang 9 ). Studies on HIV and obesity have mainly been conducted in settings where patients are treated with ART, which may confound some of the findings and interpretations, as ART has been associated with lipodystrophy and obesity( Reference Mankal and Kotler 5 ). Although increased population obesity rates have been documented in countries with limited resources including those in Southern Africa( Reference Gersh, Sliwa, Mayosi and Yusuf 10 ), studies on the relationship between HIV and obesity in African settings are lacking. It has been estimated that in sub-Saharan Africa, 10 % of women have a BMI≤18·5 kg/m2, with wasting still prevalent in some areas( Reference Uthman 11 ). However, the general population in sub-Saharan Africa is experiencing a nutrition-related transition with a rapid rise in being overweight and obese( Reference Popkin, Adair and Ng 12 ).
Economic growth, urbanisation and diminished physical activity are contributing to the rise of obesity in Southern Africa( Reference Walker, Adam and Walker 13 ). Studies of obesity and its effect on HIV disease progression have so far been inconclusive, with some reporting an association and others no association between cluster of differentiation 4 (CD4) cell counts and obesity( Reference Crum-Cianflone, Roediger and Eberly 6 , Reference Shor-Posner, Campa and Zhang 9 , Reference Adeyemi, Vibhakar and Evans 14 – Reference Shuter, Chang and Klein 16 ). Botswana is also experiencing an increase in overweight and obesity in the general population( Reference Letamo 17 ). The prevalence of overweight/obesity in adult males is estimated to be 21·5/5·8 % and adult females 52·6/24·1 %, respectively( Reference Ng, Fleming and Robinson 18 ).
Botswana has one of the world’s worst HIV epidemics, with a prevalence rate of 25·2 %, among men and women aged 15–49 years( 19 ), and it has been one of the first developing countries to provide universal access to ART( Reference Bussmann, Wester and Thomas 20 ). Countries, especially in sub-Saharan Africa, however, are still facing many challenges in providing ART and maintaining adherence( Reference De Cock and El-Sadr 21 ). Therefore, having information on nutrition-related measures and their impact on delaying disease progression, in those regions where these two epidemics are co-occurring, is timely.
The objective of this study was to examine the relationship of overweight and obesity (BMI≥25 kg/m2) with HIV disease progression in HIV+ asymptomatic adults who were ART naïve and had CD4 cell counts>350 cells/µl over 18 months in Botswana. The primary outcome for this subanalysis of a prospective study was time to the first occurrence of a 25 % or greater decrease in CD4 cell count during the course of the study, and the secondary outcomes were time to reaching CD4 cell count<250 cells/µl, AIDS-defining conditions and changes in HIV viral load.
Methods
Study design and participants
This study was a subanalysis of data from a prospective cohort study that included 219 HIV-seropositive men and women from the placebo group of a clinical trial of nutritional supplementation in Botswana. The parent study, from which the placebo group for the current observational study was drawn, was a multifactorial, randomised, double-blinded, placebo-controlled clinical trial, which investigated whether supplementation with multivitamins and Se could improve immune function and prolong time to AIDS in ART-naïve, HIV-seropositive adults( Reference Baum, Campa and Lai 22 ). The placebo group was used for this study to avoid any confounding effects from the micronutrient supplementation on the outcome measures. Participants were eligible for the parent study with documentation of HIV-seropositive test results, CD4 cell count>350 cells/µl, BMI>18 kg/m2 for women and >18·5 kg/m2 for men, age≥18 years, not on ART, lack of a past history of AIDS-defining conditions or participation in another clinical trial. Women of child-bearing potential were tested for pregnancy before randomisation and were excluded if pregnant or became pregnant during the study. According to the current Botswana guidelines, HIV-seropositive adults receive ART when their CD4 cell counts are <350 cells/µl. The data for this study were collected between 2004 and 2009. When the parent study began recruitment, ART was initiated when the patient CD4 cell count reached 200 cells/µl. Following the new guidelines, in March 2008, it was changed to 250 cells/μl. At this point, the changes were submitted to the respective Institutional Review Board (IRB) and funding agency, and the new outcome was adjusted statistically. All participants were recruited from the Botswana–Harvard AIDS Institute Partnership in Gaborone, Botswana. Participants received their medical follow-up in the same clinic where this study was conducted. The Florida International University IRB, the Harvard School of Public Health IRB and the Botswana Health Research Unit of the National Ministry of Health approved the study. Appropriate informed consent was obtained, and clinical research was conducted in accordance with guidelines for human experimentation as specified by the US Department of Health and Human Services and the researchers’ institutions.
Clinical data
At baseline and every 3 months, physical examination was carried out and medical history was obtained by a trained nurse or a physician. Anthropometrics were obtained, and BMI was calculated by dividing the weight in kilograms by height in metres squared. Bioelectrical impedance analysis (BIA) using the Biodynamics body composition analyzer (model BIA-310; Biodynamics Corp.) was used to estimate fat and fat-free mass. Fat-free mass and fat mass are estimated with a regression equation based on data obtained through comparison between bioimpedance estimates with hydrodensitometry (Biodynamics, Inc.). The equation used was fat-free mass=(a×Ht2)+(b×Wt)+(c×A)+(d×R)+e, where Ht is height in centimetres, Wt is weight in kilograms, A is age in years and R is impedance (Ω). The constants, a through e, are proprietary information of Biodynamics, Inc. Electrodes were placed on the participant’s right hand and wrist and right foot and ankle. Blood pressure was measured in the left arm with the elbow flexed to heart level. Subjects were measured without shoes and socks. Morbidity information including AIDS-defining conditions was assessed using questionnaires that were developed and validated by the Botswana–Harvard Partnership, and used in previous studies( Reference Wester, Koethe and Shepard 23 ), at screening and at every monthly visit, and confirmed by documentation in the medical chart. These questionnaires captured the diagnosis and/or signs and symptoms if they were detected during study visit. The date of onset and date of resolution if applicable were also recorded and confirmed by review of the medical chart.
Laboratory data
At baseline and every 3 months, blood samples were collected to determine CD4 and cluster of differentiation 8 (CD8) cell counts. Every 6 months, blood was also drawn to evaluate HIV-1 RNA load, as well as for a lipid panel and blood chemistry. Lymphocyte phenotype was determined using a four-colour immunophenotyping panel of monoclonal antibodies. Differential counts were determined using a Coulter MaxM hematology instrument and corroborated with cytocentrifuge smears. HIV-1 RNA load was determined using an in vitro nucleic acid amplification test (Amplicor reagents and protocol; Roche Diagnostics).
Nutritional data
24 h-dietary recalls were collected at baseline and every 3 months by trained clinical staff. Macronutrient and fibre intakes were calculated using the NutriBase Professional, version 9 (Cybersoft) programme modified for the South Africa database, including native foods for which information was available. An average of at least three dietary recalls was used to obtain estimated energy, carbohydrate, protein, fat and fibre intakes.
Statistical analysis
BMI was stratified into two groups: normal weight (BMI=18·0–24·9 kg/m2) and overweight/obese (BMI≥25 kg/m2). The overweight and obese BMI categories were combined to increase the sample size for the analyses. Laboratory markers of HIV infection and disease progression included in the analyses were absolute CD4 cell count and CD4% of total lymphocytes, absolute CD8 cell count and CD8% of total lymphocytes, CD4:CD8 ratio and HIV-1 RNA load. Descriptive statistics including frequencies, percentages, medians and interquartile ranges were used to characterise the study variables (primary and secondary outcomes) at baseline and the differences between the two BMI groups. Mann–Whitney U and χ 2 tests were used to determine differences in HIV disease progression and markers of nutritional status between the BMI groups and by sex. Linear and logistic regressions at baseline and 18 months were conducted to observe the relationship between BMI and HIV disease progression and body composition measures from BIA using BMI as a continuous and dichotomous variable. Variance inflation factors (VIF) were used to check for multicollinearity. All models had a VIF<5; therefore, multicollinearity did not affect the results. Proportional survival hazard models were used to compare hazard ratios (HR) on time to events of HIV disease progression outcomes with BMI groups, with BMI as a continuous variable and with body composition over 18 months. The primary outcome for this subanalysis was time to the first occurrence of a 25 % or greater decrease in CD4 cell count during the course of the study and secondary outcomes were time to CD4 cell count<250 cells/µl, and AIDS-defining conditions. These events were documented from the time of inclusion into the study to the date of the visit in which the event was recorded. All models were adjusted for covariates. For all analyses, a two-sided test was used, and P<0·05 was considered to be statistically significant. Statistical analyses were carried out using SPSS version 21.
Results
Demographics and clinical characteristics
The parent study randomised 878 participants in four supplementation groups in a factorial design. For this longitudinal secondary analysis of data, we only analysed the placebo group, to prevent confounding our results with the use of active supplementation received by the other three arms of the parent study. A total of 219 participants were randomly assigned into a placebo group by the parent study( Reference De Cock and El-Sadr 21 ). Two participants had missing data and were excluded from the final analysis. In all, seventy-eight participants at baseline had BMI≥25 kg/m2 and 139 had BMI of 18·0–24·9 kg/m2. Most of the participants were female (75·1 %) with a median age of 33 years, reflecting the characteristics of the epidemic in Botswana( 24 ). Almost half (45·2 %) of the participants had at least a secondary education level and most of them had children (85·7 %) (Table 1).
Table 1 Demographic characteristics by BMI groups at baseline (Medians and interquartile ranges (IQR), numbers and percentages)

CD4, cluster of differentiation 4; CD8, cluster of differentiation 8.* Statistically significant (P<0·05).
† The currency in Botswana is pula and 1 pula was equivalent to about $0·15–0·22 USD at the time the study was conducted.
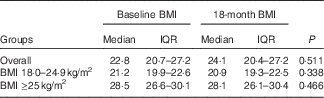
There were differences in demographic factors, serum lipids, glucose and blood pressure among the BMI groups. Those with BMI≥25 kg/m2 were older and more likely female, married, had children, and had higher levels of total cholesterol, TAG, LDL-cholesterol, glucose and blood pressure (Table 1). There were no differences in laboratory markers of HIV infection at baseline between the BMI groups. Total dietary energy intake and physical activity were not statistically different between the BMI groups. BMI were stable throughout the 18 months of follow-up and did not statistically change within the normal and overweight/obese groups (Table 2).
Table 2 Change in BMI (kg/m2) from baseline to 18 months by BMI groups (Medians and interquartile ranges (IQR))
BMI, body composition and laboratory markers of HIV infection and disease progression
Logistic regression analyses that compared the BMI groups at baseline, 18 months and the change between these visits in the laboratory markers of HIV infection and disease progression were conducted; however, no significant associations were found with markers of HIV disease progression.
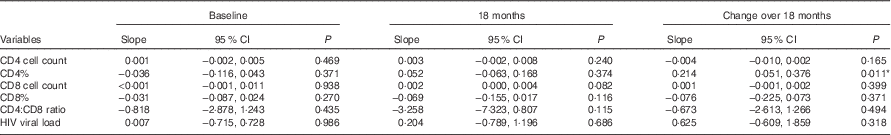
Linear regressions were also conducted to compare the measures of HIV disease progression and BMI as a continuous variable at baseline, 18 months and the change between these visits (Table 3). The change in CD4% between baseline and 18 months was significantly associated with BMI, showing that for every unit increase in BMI there was 0·214 increase in the difference in CD4% (β 0·214; 95 % CI 0·051, 0·376; P=0·011). The association between CD8 cell count and BMI at 18 months approached significance (β 0·002; 95 % CI 0·000, 0·004; P=0·082). All models were adjusted for age, sex, marriage and children.
Table 3 Linear regression models of the relation between continuous BMI and measures of HIV disease progression† ‡
* Statistically significant (P<0·05).
† Individual models analysed for each independent variable and adjusted for age, sex, marriage and children.
‡ Dependent variable for baseline analysis included continuous BMI at baseline, dependent variable for 18 months of analysis included continuous BMI at 18 months, and dependent variable for change over 18 months of analysis included continuous BMI at baseline.
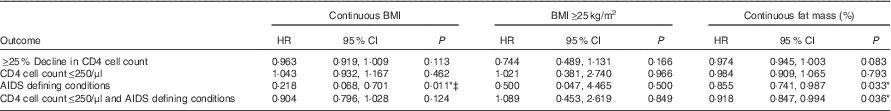
Cox proportional hazard model with BMI as a dependent variable showed that higher baseline BMI was associated with a significantly lower risk of having an AIDS-defining condition (HR 0·218; 95 % CI 0·068, 0·701; P=0·011), after adjusting for age, sex, marriage, children and baseline CD4 cell count and viral load. Significant associations were not found between continuous BMI and ≥25 % decline in CD4 cell count, CD4 cell count≤250/μl and the combined outcome of CD4 cell count≤250/μl and/or AIDS-defining conditions, whichever occurred first. In addition, BMI≥25 kg/m2 as compared with those with BMI of 18–24·9 kg/m2 was also not found to have significant associations with the outcomes of ≥25 % decline in CD4 cell count (HR 0·744; 95 % CI 0·489, 1·113; P=0·166), CD4 cell count≤250/μl (HR 1·021; 95 % CI 0·381, 2·740; P=0·966), AIDS-defining conditions (HR 0·500; 95 % CI 0·047, 4·465; P=0·500) and the combined outcome of CD4 cell count≤250/μl and/or AIDS-defining conditions (HR 1·089; 95 % CI 0·453, 2·619; P=0·849) (Table 4).
Table 4 Effect of baseline body composition on HIV disease progression outcomes in HIV+ adults in Botswana during a follow-up period of 18 monthsFootnote † (Hazard ratios and 95 % confidence intervals)
CD4, cluster of differentiation 4; CD8, cluster of differentiation 8.* Statistically significant (P<0·05).
† Cox proportional hazards model were used to examine the effect of baseline continuous BMI, the effect of BMI groups (0=BMI 18·0–24·9 kg/m2 and 1=BMI≥25 kg/m2), and baseline continuous fat mass % on individual HIV disease progression outcomes. All individual HIV disease progression outcomes were analysed as separate models and adjusted for age, sex, marriage, children and baseline CD4 count and viral load.
‡ Model adjusted for age, sex, children and baseline CD4 cell count and viral load.
Cox proportional hazard models showed that higher fat mass at baseline was significantly associated with a decreased risk of having an AIDS-defining condition (HR 0·855; 95 % CI 0·741, 0·987; P=0·033) and a decreased risk of the combined outcome of CD4 cell count≤250/μl and/or AIDS-defining conditions, whichever occurred first (HR 0·918; 95 % CI 0·847, 0·994; P=0·036). A trend towards significance was seen with higher fat mass association with a lower risk of 25 % decline in CD4 cell count (HR 0·974; 95 % CI 0·945, 1·003; P=0·083) (Table 4).
Sensitivity analyses
Sensitivity analyses were performed to examine the effect of BMI with exclusion of the lower end of the normal BMI range (18–20 kg/m2) on measures of HIV disease progression. The Cox proportional hazard models were re-analysed excluding participants with BMI of 18–20 kg/m2. BMI and fat mass no longer had significant associations with measures of HIV disease progression over 18 months. However, BMI≥25 kg/m2 was protective of the event combining the outcomes of CD4 cell count≤250/μl, and/or AIDS-defining conditions (HR 0·219; 95 % CI 0·055, 0·873; P=0·031) when compared with BMI of 18–20 kg/m2 after adjusting for age, sex, marriage, children and baseline CD4 count and viral load.
Discussion
This study showed that higher baseline BMI and fat mass were associated with delaying the time to the first AIDS-defining condition in HIV-seropositive, ART-naïve adults in Botswana during 18 months of follow-up. Higher baseline BMI was associated with significantly lower risk of having an AIDS-defining condition. Higher fat mass at baseline was also significantly associated with a decreased risk of having AIDS-defining conditions and the combined outcome of having CD4 cell count≤250/µl and/or AIDS-defining conditions, or whichever occurred earlier. These results are in agreement with the earlier observation by Shor-Posner et al. ( Reference Shor-Posner, Campa and Zhang 9 ) who found that obese study participants in Miami, FL, were significantly less likely to have a 25 % decline in CD4 cell count over 18 months. Wanke et al. ( Reference Wanke, Silva and Ganda 25 ) also demonstrated that a past incidence of more than one AIDS-defining condition was associated with 1·3-fold risk of having BMI<20 kg/m2, and AIDS-defining conditions were also associated with higher risk of wasting.
Higher BMI was also associated with a higher CD4% after 18 months of follow-up. CD4% is considered to provide better prognostic information before ART is initiated( Reference Taylor, Fahey and Detels 26 – Reference Hulgan, Raffanti and Kheshti 28 ). Other investigators have also found that immune counts are affected by weight and/or BMI in HIV-infected adults( Reference Adeyemi, Vibhakar and Evans 14 , Reference Crum-Cianflone, Roediger and Eberly 29 ). Crum-Cianflone et al. ( Reference Crum-Cianflone, Roediger and Eberly 29 ) compared normal weight adults with obese HIV-seropositive adults at diagnosis and through highly active antiretroviral therapy (HAART) or ART initiation and concluded that those who were obese had smaller reductions in CD4% through time, regardless of whether the diagnosis took place in the pre-HAART era or afterwards. Interestingly, Crum-Cianflone et al.( Reference Crum-Cianflone, Roediger and Eberly 29 ) also showed that weight did not have an effect on immune cells, including CD4 cell counts at HIV diagnosis, but instead the effect of weight on immune cells became more significant as the HIV disease progressed. Our study also did not find any significant differences in absolute CD4 cell count or CD4% at baseline between the normal-weight and overweight/obese groups, possibly because the participants were recruited relatively early in the disease.
This is the first study that demonstrated a longitudinal association between higher fat mass and lower risk of AIDS-defining conditions. Higher fat mass was associated with a lower risk of the combined outcome of CD4 cell count≤250/μl and/or AIDS-defining conditions, whichever occurred first. These findings are in agreement with previous cross-sectional studies that found that women with previous AIDS-defining conditions had lower total body fat( Reference Visnegarwala, Raghavan and Mullin 30 ) and that BMI was positively associated with CD4 cell count among HIV-seropositive women, which was hypothesised to be due to excess fat( Reference Jones, Hogan and Snyder 15 ).
Although in the survival analysis a positive association between CD4 cell count and fat mass approached significance, our earlier cross-sectional analysis using the entire cohort (n 878) at baseline showed that this relationship was statistically significant( Reference Riedel, Ramamoorthy and Baum 31 ). Thus, our findings suggest that higher BMI and higher fat mass were associated with slower disease progression in ART-naïve, HIV-infected adults. We hypothesise that high HIV viral load and immune activation in patients who are not on ART are associated with a hypermetabolic state( Reference Melchior 32 ) requiring additional energy( Reference Schaible and Kaufmann 33 , Reference Scrimshaw and SanGiovanni 34 ); thus, patients who are obese may have an advantage in maintaining and utilising fat stores to assist in preserving the immune system( Reference Shuter, Chang and Klein 16 , Reference Chandra 35 ). Moreover, recent research has shown that adipocyte-derived adipokines affect a range of cellular immune processes and may also be involved in preserving the CD4 cell count( Reference Koethe, Hulgan and Niswender 36 ). On the other hand, maintaining a large lean body mass has higher energy costs, and stored protein is less available and provides less energy than the same weight in fat( Reference Berdanier 37 ). Thus, this might explain why lean body mass was not protective in this cohort who were not on ART.
Interestingly, a recent study utilising data from 8381 participants in the North American AIDS Cohort Collaboration on Research Design has found that higher BMI (approximately 30 kg/m2) and presumably higher amount of body fat at ART initiation was associated with greater CD4 cell recovery at 12 months, showing that the beneficial effect of higher BMI was largely preserved after the patients initiated ART( Reference Koethe, Jenkins and Lau 38 ). However, once patients control their HIV viral load with ART, the energy requirements are most likely reduced and classic risk factors for chronic disease develop( Reference McGee 39 – Reference Calle, Rodriguez and Walker-Thurmond 42 ). The participants of this study in the overweight/obese group had higher levels of total cholesterol, LDL-cholesterol, TAG, glucose and blood pressure, although the differences in these parameters were not clinically significant between the BMI groups. These differences could, over a period of time, potentially increase their risks for chronic conditions.
Stevens et al.( Reference Stevens, Bradshaw and Truesdale 43 ) have stated concerns over providing confusing messages when discussing the obesity paradox and the possible harm it will have on public health efforts related to reducing obesity rates to lower the risk of chronic diseases. Conversely, other researchers( Reference Dixon, Egger and Finkelstein 44 ) theorise that our view of normal or optimal weight or BMI categories are too strict and may be biologically unsuitable. In this study, a BMI of 18–20 kg/m2, which is considered to be in the normal range, was still associated with HIV disease progression and may need to be further examined to see whether for HIV infection a shift in the BMI categories might be more appropriate. They also argue that those with an established disease diagnosis may benefit from an emphasis on lifestyle recommendations regarding physical activity and nutrition rather than intentional weight loss or gain. Important nutritional elements to consider for people living with HIV may include medication–nutrition interactions, barriers to maintenance and restoration of nutritional status, food and water safety, food security issues and evaluation of interactions with alcohol and illicit drugs( Reference Fields-Gardner and Campa 45 ).
The strengths of this study consist of capturing and analysing longitudinal data from ART-naïve, HIV-seropositive participants in Botswana, which were collected from the early asymptomatic stage through defined health outcomes. During the time this study was conducted, ART initiation was at 200 cells/µl and it was later changed to 250 cells/μl following the WHO recommendations. Following the current Botswana guidelines, HIV-seropositive adults receive ART when their CD4 cell counts fall below 350 cells/µl. Although present WHO guidelines recommend initiation of ART at CD4 cell count of 500 cells/µl, most countries affected by HIV have limited resources that may affect the ability to obtain this standard( 46 , Reference Piot and Quinn 47 ). The information gathered in this study is needed and is timely as countries especially in sub-Saharan Africa are still having many challenges in providing ART and maintaining adherence( Reference De Cock and El-Sadr 21 ).
The limitations of this study include its observational nature. Moreover, with fewer participants in the higher BMI group, we were unable to examine differences between overweight and obese groups. Underweight participants were not included in the parent study, as underweight is a strong prognostic indicator of AIDS( Reference De Cock and El-Sadr 21 ). However, from the sensitivity analyses, BMI of 18–20 kg/m2 was associated with significant HIV disease progression outcomes. In addition, these findings can only be generalised to HIV-seropositive patients in Africa who are not on ART. However, having information on delaying disease progression in the setting of sub-Saharan Africa is timely.
In summary, the results of this study demonstrate that higher BMI and fat mass were protective and delayed HIV disease progression among HIV-seropositive, ART-naïve adults in Botswana. Higher baseline BMI was associated with significantly lower risk of having an AIDS-defining condition. Higher fat mass at baseline was also significantly associated with a decreased risk of having AIDS-defining conditions and/or the combined outcome of having CD4 cell count≤250/µl and AIDS-defining conditions, whichever occurred earlier. Mechanistic studies on the relationship between BMI and body composition on disease progression are needed to clarify the obesity paradox in HIV.
Acknowledgements
The authors thank the participants of the study without whom advancement in the nutritional management of the HIV disease would not be possible and the Botswana–Harvard Partnership, which enabled the conduct of this study. The authors thank Jag Khalsa, PhD, Medical Consequences Branch, in the Division of Pharmacotherapies and Medical Consequences of Drug Abuse at the National Institutes on Drug Abuse, NIH, for his leadership, advice and support.
This work was supported by the National Institute on Drug Abuse (R01-DA-016551). The National Institute on Drug Abuse had no role in the design, analysis or writing of this article.
M. K. B. and R. M. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: M. K. B., A. C., M. E., R. M., S. S. M.; acquisition of data: A. C., S. S. M., H. B., S. M., J. M., R. M.; analysis and interpretation of data: A. C., M. K. B., H. B., M. E., R. M.; drafting of the manuscript: S. S. M.; critical revision of the manuscript for important intellectual content: M. K. B., A. C., H. B., M. E., R. M., O. D. W., F. G. H.; statistical analysis: S. S. M., O. D. W.; obtained funding: M. K. B., A. C., M. E., R. M.; administrative, technical, or material support: M. K. B., A. C., S. S. M., J. M., M. E., R. M.; study supervision: A. C., H. B., J. M., R. M., M. K. B.









