Depression is a common cause of disability( Reference Vos, Flaxman and Naghavi 1 ). In the USA, depression cost an estimated $210·5 billion in 2010 and resulted in almost 400 million disability days during 2014( Reference Greenberg, Fournier and Sisitsky 2 ). There is increasing interest in whether lifestyle factors such as diet can prevent or treat depression( Reference Sarris, O’Neil and Coulson 3 ). A recent meta-analysis concluded that high fish consumption could reduce the risk of depression( Reference Li, Liu and Zhang 4 ). This raises the question of which component(s) of fish are responsible for the protective effect( Reference Evans 5 ).
One candidate is the n-3 PUFA, particularly the long-chain EPA and DHA, which are highly available in fish( Reference Nichols, Petrie and Singh 6 ), and DHA is highly concentrated in the brain( Reference Youdim, Martin and Joseph 7 ). The role of n-3 PUFA in neurophysiology and depression has been investigated extensively( Reference Mello, Gassenferth and Souza 8 , Reference Deacon, Kettle and Hayes 9 ), and there is evidence suggesting that long-chain n-3 PUFA may be beneficial in the prevention and treatment of depression( Reference Deacon, Kettle and Hayes 9 , Reference Appleton, Rogers and Ness 10 ), although the mechanism is debated( Reference Mello, Gassenferth and Souza 8 ) and there have not been enough randomised controlled trials with sufficient power to demonstrate this conclusively( Reference Appleton, Sallis and Perry 11 ). The ratio of n-6 PUFA to n-3 PUFA in erythrocytes has also been associated with depression in some studies( Reference Sontrop and Campbell 12 ).
Another component of fish suggested to be beneficial for mental health is tyrosine( Reference Evans 5 ), an amino acid that is particularly abundant in some fish relative to other common forms of dietary protein( Reference Tyrrell 13 ). Tyrosine is a precursor to dopamine, catecholamines, melanins and thyroid hormones( Reference Tyrrell 13 ). Irregularities in these systems have been associated with neurophysiological and psychological problems, including depression( Reference Dailly, Chenu and Renard 14 – Reference Jackson 16 ). Acute tyrosine depletion in healthy volunteers has been linked to unipolar depression( Reference McLean, Rubinsztein and Robbins 17 ), and chronic low levels of tyrosine in the brain have been associated with depression in longitudinal studies of phenylketonuria( Reference Clacy, Sharman and McGill 18 ). Tyrosine supplementation has been studied as a possible treatment for depression with mixed results( Reference Gelenberg and Gibson 19 ). There has only been one randomised double-blind trial; that study found that tyrosine supplementation was not an effective treatment for depression( Reference Gelenberg, Wojcik and Falk 20 ).
We previously reported that, among women, higher baseline fish consumption was associated with a lower risk of a new depressive episode during the 5-year follow-up, an association that remained robust when subjects with a history of depression were excluded( Reference Smith, Sanderson and McNaughton 21 ). Since publishing that paper, we have conducted metabolomics analysis on stored blood samples. This study investigates whether the protective association between fish consumption and depression observed among young Australian women is mediated by n-3 fatty acid status or tyrosine status.
Methods
Study population
The Childhood Determinants of Adult Health (CDAH) study is a follow-up to the 1985 Australian Schools Health and Fitness Survey, a nationally representative sample of 7- to 15-year-old Australian schoolchildren (n 8498). Participants were traced during 2001–2002 and 5170 enrolled in the CDAH study( Reference Gall, Jose and Smith 22 ). During 2004–2006, 2410 participants attended one of thirty-four study clinics held around Australia for physical measurements, aged 26–36 years (baseline for this analysis), and completed questionnaires. The majority of these participants (64 %) gave a fasting blood sample, and the characteristics of this subset are similar to the full set of participants (data not shown). In a second follow-up conducted 5 years later (2009–2011), participants completed written questionnaires and a telephone interview (follow-up).
The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the Southern Tasmanian Health and Medical Ethics Committee. Written informed consent was obtained from all participants.
Fish consumption
Fish consumption was assessed at baseline via a 127-item FFQ( Reference Smith, McNaughton and Gall 23 ), with nine items relating to fish and seafood (canned fish, fresh fish, frozen fish, fried fish, mussels/oysters, lobster/crayfish/yabbies (small lobsters), calamari/squid, prawns and other seafood)( Reference Smith, Sanderson and McNaughton 21 ). The FFQ was based on a validated questionnaire developed for Australian adults( Reference Ireland, Jolley and Giles 24 , Reference Hodge, Patterson and Brown 25 ) and was a slightly modified version of the one used in the 1995 Australian National Nutrition Survey( Reference McLennan and Podger 26 ). Participants were asked to recall how frequently they consumed each item over the previous 12 months by selecting one of nine response items, ranging from ‘never/less than once a month’ through to ‘6 or more times a day’. Weekly equivalents were calculated on the assumption that one serving was consumed on each occasion( Reference Smith, McNaughton and Gall 23 ), and then summed to determine the total weekly consumption of fish and seafood (hereafter ‘weekly fish consumption’). Participants who did not answer all of the fish questions were excluded from the analysis.
Depression
At follow-up, participants completed the computerised lifetime version of the Composite International Diagnostic Interview (CIDI-Auto 2.1) via a computer-assisted telephone interview designed for use by non-clinical interviewers. Major depression or dysthymic disorder (hereafter ‘depression’) was defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). Age at the time of the most recent episode was used to determine whether an episode had occurred since baseline. Participants who had a new episode of depression during the 5-year follow-up were compared with those who did not have a new episode.
Metabolomics
Fasting blood samples collected at baseline were stored at −80°C for 11–13 years before being analysed using the Computational Medicine metabolomic platform( Reference Würtz, Kangas and Soininen 27 , Reference Soininen, Kangas and Wurtz 28 ), which used high-throughput NMR spectroscopy to profile the serum levels of 233 key metabolic markers. All samples were subjected to automated quality control, and values for any metabolic markers that could not be extracted reliably were excluded from the analysis( Reference Würtz, Kangas and Soininen 27 , Reference Soininen, Kangas and Wurtz 29 ). Here we focus on tyrosine, DHA, total n-3 PUFA (including long-chain and short-chain n-3 PUFA) and total PUFA (including both n-3 and n-6 PUFA). We also examined the n-3:n-6 ratio, defined as (total n-3 PUFA)/(total n-6 PUFA), which includes both long- and SCFA. Although n-6:n-3 is the ratio more commonly used, we use the inverse form (n-3:n-6) so that higher values are associated with greater protection. In addition, we examined the ratio of tyrosine:total concentration of large neutral amino acids (LNAA), as tyrosine competes with LNAA for transport across the blood–brain barrier( Reference Demling, Langer and Wörthmüller 30 ). LNAA included tyrosine, valine, leucine and isoleucine, but data for tryptophan were not available. The CV for this technique were 2·7 % for n-3 fatty acids (2·7 % for DHA) and 7·7 % for tyrosine( Reference Kettunen, Demirkan and Wurtz 31 ).
Covariates
At baseline, participants reported their marital status, education, occupation and smoking status. Weight and height were measured at study clinics using portable digital scales (Heine) and a portable stadiometer (Invicta). Height and weight were self-reported by participants who did not visit a study clinic and a correction factor was applied( Reference Venn, Thomson and Schmidt 32 ). BMI was calculated as weight (kg) divided by the square of height (m2). Health status was assessed using the twelve-Item Short-Form Health Survey( Reference Ware, Kosinski and Keller 33 ). Information from the FFQ and food habits questionnaire was used to create a dietary guideline index (DGI), based on the Australian Guide to Healthy Eating( Reference Smith, Schmerlaib and Kellett 34 ) and the Dietary Guidelines for Australian Adults( 35 ). The DGI included fifteen components, scored from 0 to 10, with 10 indicating optimal intake (total range, 0–150). The DGI has been shown to be a valid measure of diet quality( Reference McNaughton, Ball and Crawford 36 , Reference McNaughton, Dunstan and Ball 37 ).
Statistical analysis
For each metabolic marker of interest, mediation was assessed using the four steps of mediation proposed by Baron & Kenny( Reference Baron and Kenny 38 ). This method tests potential mediators by examining the pathways from the independent variable to the mediator and from the mediator to the dependent variable, as well as considering the effect of controlling for the mediator on the pathway from the independent variable to the dependent variable. The four steps are as follows: (1) test whether the independent variable is associated with the dependent variable; (2) the independent variable is associated with the mediator; (3) the mediator variable is associated with the dependent variable; and (4) establish that the mediator variable mediates the association between the independent and dependent variables. In the first step, we tested the direct pathway between fish consumption (independent variable) and a new depressive episode (dependent variable) to verify the association established in our earlier study( Reference Smith, Sanderson and McNaughton 21 ). For the second step, we tested the individual associations between fish consumption (independent variable) and each metabolic marker (potential mediators), examining fish consumption as both a dichotomous and a continuous variable. For the third step, we tested the pathway from each metabolic marker (potential mediator) to depression (dependent variable), using log-binomial regression in each case. Finally, for the fourth step, we used the STATA binary_mediation command to estimate the indirect effects due to each potential mediator, and calculated this indirect effect as a proportion of the total effect. Internally, the binary_mediation command uses linear regression for continuous variables and (by default) logistic regression for categorical variables. Note that for the last three steps separate models were tested for each individual potential mediator.
For metabolites that passed all four mediation tests, we conducted further sensitivity analyses by including additional covariates in each model. Specifically, we included the same covariates as the earlier study: marital status, smoking status, weight status and self-rated health( Reference Smith, Sanderson and McNaughton 21 ). To determine whether diet quality was a confounder, we also included PUFA supplementation, the DGI score and a modified DGI score (which excluded fish items) in separate sensitivity analyses.
To determine whether the mediation effect of n-3 fatty acids and tyrosine differed by smoking status, we included a multiplicative interaction term between smoking status and fish intake. We found no evidence for interaction by smoking status, with all interactions terms P>0·33.
This study focuses on women, as our earlier study found that fish consumption was not associated with depression among men. STATA software (2012, version 12.1; StataCorp) was used for all analyses.
Results
In total, 1552 women had data on baseline fish consumption. We excluded those who did not complete the CIDI-Auto 2.1 interview (n 627), were missing covariate data (n 71), did not give a blood sample (n 304) or were missing values for tyrosine (n 4). The baseline characteristics of the 546 participants included in the analysis are shown in Table 1.
Table 1 Baseline characteristics among Australian women in the Childhood Determinants of Adult Health Study, stratified by depression status during the 5-year follow-up (Numbers and percentages; mean values and standard deviations)
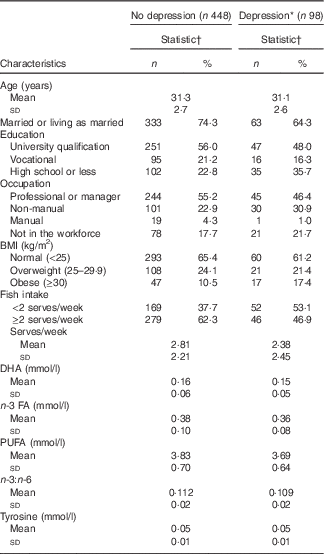
FA, fatty acids.
* New episode of depression in the preceding 5 years, as diagnosed by the Composite International Diagnostic Interview (Composite International Diagnostic Interview-Auto 2.1).
† Sample sizes vary owing to missing data (range 442–448 for women without depression; 97–98 for women who had a depressive episode).
In total, ninety-eight women had a depressive episode during the 5-year follow-up. Although the study population was smaller than our previous study( Reference Smith, Sanderson and McNaughton 21 ), as not all participants gave a blood sample, a similar association between fish consumption and depression was found (7·6 % lower risk of a new depressive episode with every additional serve of fish), verifying Step 1 of the Baron and Kenny mediation tests.
The results for the three remaining tests are summarised in Table 2 in descending order of mediation, for the key metabolic markers of interest, and in the online Supplementary Tables for all metabolites. DHA was the only metabolic marker to pass all three tests. DHA levels were positively associated with fish consumption (continuous: β=0·005, P<0·001; dichotomous: β=0·025, P<0·001, Table 2) and negatively associated with depression (β=−3·87, P=0·03). As a proportion of the total effect, the indirect effect due to DHA was the highest of all metabolites examined, estimated at 25 % (continuous) and 17 % (dichotomous). The associations remained in the sensitivity analyses when covariates were added to the models.
Table 2 The mediation effect of n-3 fatty acids and tyrosine on the association between fish consumption at baseline and new episodes of depression in the 5-year follow-up
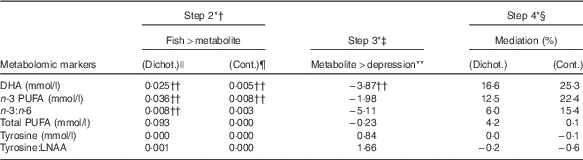
Cont, continuous; Dichot, dichotomous; LNAA, large neutral amino acids.
* Mediation steps proposed by Baron & Kenny( Reference Baron and Kenny 38 ).
† Step 2: effect size of association between fish consumption (independent variable) and metabolic marker (dependent variable). The reported values are β-coefficients.
‡ Step 3: effect size of association between metabolic marker (independent variable) and new episodes of depression during the 5-year period (dependent variable). The reported values are β-coefficients.
§ Step 4: proportion of total effect mediated by metabolic marker when included as a covariate in a model with fish consumption (independent variable) and depression (dependent variable), estimated using the binary_mediation STATA command.
|| Dichot: fish consumption treated as a categorical variable, comparing women who ate fish at least two times per week (n 325) at baseline compared with women who consumed fish less than two times per week (n 221).
¶ Cont: fish consumption treated as a continuous variable (serves per week).
** Ninety-eight participants had a new depressive episode during the 5-year follow-up and 448 did not.
†† Statistically significant at P<0·05.
Tyrosine levels and the tyrosine:LNAA ratio were also positively associated with fish consumption, although the effect sizes were very small and NS (β<0·001, P=0·83 for tyrosine and β<0·001, P=0·57 for tyrosine:LNAA, Table 2). However, rather than being protective against depression, higher levels of tyrosine and the tyrosine:LNAA ratio were associated with a higher risk of depression, but again these associations were not significant (β=0·84, P=0·92 for tyrosine and β=1·66, P=0·75 for tyrosine:LNAA). These associations were not sustained during sensitivity testing when PUFA supplementation and the covariates from the original study were included in the model. Tyrosine (but not tyrosine:LNAA) passed all three mediation tests, but the proportion of the total effect mediated by tyrosine was <1 %.
Discussion
This study builds on our previous work that reported higher baseline fish consumption was associated with a lower risk of a new depressive episode among young women( Reference Smith, Sanderson and McNaughton 21 ). In this study, we have shown that the association is partially mediated by baseline plasma levels of DHA but not tyrosine.
DHA only partially explained the protective role of fish against depression, suggesting that there may be other components in fish that are protective or there may be synergistic effects between the nutrients in fish. The mediation proportion for DHA was roughly three to four times higher than the highest non-n-3 fatty acid-related metabolites. DHA passed all three steps of the Baron and Kenny method for all models tested, including models with smoking status and use of fish oil supplements as covariates. We did not adjust for age or physical activity, as these variables were not associated with depression in this sample and therefore cannot be confounders( Reference Smith, Sanderson and McNaughton 21 ), but other unmeasured confounding is possible.
In the current study, total n-3 PUFA and the n-3:n-6 ratio did not satisfy all criteria for mediation. The mediation effect for n-3 PUFA was lower than that of DHA alone, probably because the effect of DHA is diluted by the inclusion of n-3 PUFA that do not have a protective effect against depression, such as α-linolenic acid.
To our knowledge, no previous studies have conducted mediation analysis to identify the components in fish that mediate the protective effect of fish on depression. Although we do not have a measure for EPA in our study, our findings add to the previous literature examining associations between plasma/serum or dietary n-3 fatty acids and depression. A meta-analysis of fourteen studies found that DHA, EPA and n-3 fatty acid levels in plasma/serum were lower among patients with depression than controls( Reference Li, Liu and Zhang 4 ). A cross-sectional study in Japanese adults (40+ years) found an inverse association between serum DHA and EPA levels and depressive symptoms( Reference Horikawa, Otsuka and Kato 39 ). Dietary intake of DHA and EPA was found to be inversely associated with the risk of depression symptoms after a 3-year follow-up in the Coronary Artery Risk Development in Young Adults (CARDIA) study( Reference Colangelo, He and Whooley 40 ). In contrast, several longitudinal studies have reported no association between dietary intake of n-3 PUFA and depression( Reference Hakkarainen, Partonen and Haukka 41 – Reference Lucas, Mirzaei and O’Reilly 43 ). The conflicting results may reflect the different methods used to assess fish intake and depression, different ages of the samples (e.g. older adults or having a wide age range) and unmeasured or residual confounding.
n-3 Fatty acid supplements have been tested in randomised controlled trials as a possible treatment of depression, with inconsistent results. Two recent meta-analyses published in 2016 have conflicting conclusions. A Cochrane systematic review and meta-analysis of twenty randomised controlled trials found insufficient evidence to support the use of n-3 fatty acid supplements as a treatment for depression( Reference Appleton, Sallis and Perry 11 ). Most studies included in the review were small (all but three studies had <100 participants), and of short duration (4–16 weeks). In contrast, a meta-analysis of thirteen studies found a beneficial effect of n-3 fatty acid supplementation in patients with depression, especially for higher doses of EPA and when used in combination with antidepressants( Reference Mocking, Harmsen and Assies 44 ). However, the beneficial effect was small, and it has been argued that the effect may not be clinically important and the results were influenced by poorer-quality studies( Reference Bastiaansen, Munafo and Appleton 45 ).
There are several limitations that need to be considered. The FFQ did not distinguish between fatty and non-fatty fish, and it is possible that analysis focusing on fatty fish may reveal a stronger association with depression, as well as a higher degree of mediation by n-3 PUFA. Data for one LNAA (tryptophan) were not available, and therefore we overestimate the tyrosine:LNAA ratio. There is the possibility that some metabolites may have degraded as a result of long-term storage, but samples were subjected to quality control. Of the 1940 participants who had blood samples analysed from the CDAH study, seventeen (0·8 %) were excluded from the analysis for degraded n-3 fatty acids (eight women), and twenty-seven (1·4 %) for tyrosine (nine women). Compounds representing lipid oxidation products were not detected in any of the samples.
Another limitation was the difference in time scales between the variables being compared. Although the FFQ assesses fish consumption retrospectively over a 12-month period and depression status was determined over a 5-year period, the metabolomic data represent a snapshot of fasting plasma levels at a single moment in time. This snapshot reflects dietary intake over the previous few days, but different nutrients are metabolised at different rates and so the degree to which fasting plasma metabolite levels can be taken as a proxy for long-term consumption may vary. Plasma and erythrocytes n-3 PUFA levels have both been shown to correlate to short-term consumption( Reference Witte, Salazar and Ballester 46 ). However, tyrosine is rapidly metabolised in the body, with levels returning to baseline within a few hours after a meal( Reference Milsom, Morgan and Sherlock 47 ). Long-term consumption patterns are difficult to capture owing to tyrosine’s multiple destinations in the body, although future studies with more frequent measurements may provide a more accurate picture. Long-term fish intake appears to be relatively stable in this cohort, as sensitivity analyses in our previous study found similar results when baseline and follow-up intake was averaged and used as the exposure variable( Reference Smith, Sanderson and McNaughton 21 ). Although it would have been ideal to also examine EPA, DHA was the only long-chain n-3 PUFA for which individual levels were available.
Data on lipid mediators or metabolites derived from PUFA may help to clarify the role of PUFA in the association between fish consumption and depression. However, EPA and lipid mediators are unable to be distinguished by the NMR platform, and complementary assays such as MS may be required. In addition, we were unable to examine change in metabolites as blood samples, and therefore the metabolite data were only collected at baseline. The study population had higher representation of professionals/managers and people married or living as married than the general Australian population of similar age. Although marital status or socio-economic status may play a role in determining susceptibility of depression, these factors are unlikely to affect how serum metabolites mediate the association between diet and depression.
Major strengths of this study include the ability to compare 233 potential mediators in the same sample using a rigorous and accepted method of mediation. Depression was assessed using a diagnostic tool, considered the ‘gold standard’ in epidemiological studies.
These findings add to a growing body of evidence that suggests that DHA may have a protective role for mental health. DHA only partially explained the protective association between fish consumption and depression, suggesting that other components in fish may benefit women’s mental health.
Acknowledgements
The authors gratefully acknowledge the contribution of Marita Dalton (study project manager), all other project staff and the study participants.
This project was funded by grants from the National Health and Medical Research Council (grants 211316, 544923); the National Heart Foundation (GOOH0578); the Select Foundation (M0020771); the Tasmanian Community Fund (D0013808); and Veolia Environmental Services. C. G. M. was supported by a National Heart Foundation of Australia Future Leader Fellowship (100849); M. A.-K. by the Sigrid Juselius Foundation and the Strategic Research Funding from the University of Oulu, Finland; A. J. V. by a National Health and Medical Research Council Fellowship (APP1008299); and K. J. S. by a National Health and Medical Research Council Early Career Fellowship (APP1072516). M. A.-K. works in a Unit that is supported by the University of Bristol and UK Medical Research Council (MC_UU_12013/1). The study was sponsored by Sanitarium (Melbourne, Victoria, Australia), ASICS Ltd (Kobe, Japan) and Target Australia Pty Ltd (North Geelong, Victoria, Australia). The funding bodies had no role in the study design, analysis or writing of this article.
K. J. S., A. J. V. and T. D. designed the research. J. L. R. performed the statistical analysis with support from K. J. S., P. O. and C. G. M.. A. J. K., P. S. and M. A.-K. analysed the metabolomics data. J. L. R. and K. J. S. wrote the paper with critical input from C. G. M., P. O. and A. J. V. All authors provided critical revision of the manuscript and approved the final version.
A. J. K. and P. S. are shareholders of Nightingale Health (former Brainshake Ltd), a company offering NMR-based metabolite profiling. The other authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517002768






