The present number of diabetics worldwide exceeds 150 million and is predicted to be >300 million by the year 2025 due to the increase in sedentary lifestyles, consumption of an energy-rich diet, obesity and higher life span(Reference Lefebvre1). Obesity has increased at an alarming rate in recent years and is now a worldwide health problem, since it is a major risk factor for insulin resistance or type 2 diabetes, impairment of liver functions and even cancer, for which the social costs are incalculable(Reference Rossner2). Cardiovascular complications (e.g. hypertension and coronary artery and peripheral vascular diseases) due to the metabolic syndrome, a clustering of pathological conditions including hyperglycaemia/dyslipidaemia (resulting from type 1 and 2 diabetes) and liver steatosis/dysfunctions, are a major cause of morbidity and mortality in diabetic and obese patients(Reference Knuiman, Hung and Divitini3). Cyclophosphamide (CP) is an alkylating agent widely used to treat a variety of malignant diseases such as lymphoma, myeloma and chronic lymphocytic leukaemia(Reference de Jonge, Huitema and Rodenhuis4). However, CP intake is associated with many serious side effects including immunosuppression and dyslipidaemia, which explains why an impressive part of the morbidity and mortality in cancer patients treated with such chemotherapeutic agents is caused by infections and hyperlipidaemic cardiomyopathy(Reference Bin-Hafeez, Ahmad and Haque5–Reference Ramadan, El-Beih and Badr7). Free radicals, especially reactive oxygen species, are involved in the pathogenesis of the metabolic syndrome and CP-induced immunosuppression(Reference Mythili, Sudharsan and Sudhahar6–Reference Ramadan, El-Beih and Abd El-Ghffar8).
Due to the harmful side effects of synthetic drugs, the enormous cost and the inability of existing modern therapies to control all pathological aspects of the diabetic disorder and obesity, and the poor availability of advanced therapies for many rural populations in developing countries, many dietary supplements that have antioxidant activity and can modulate glucose homeostasis, potentially improve lipid parameters, and are less toxic than Western medicine have been recommended for the treatment of diabetes mellitus and obesity as well as their complications nowadays(Reference Ramadan, El-Beih and Abd El-Ghffar8–Reference Seo, Gweon and Lee10). In addition, a natural modulator that shows immunostimulant activity and can alleviate immunosuppressive side effects in CP-treated cancer patients is urgently needed(Reference Bin-Hafeez, Ahmad and Haque5, Reference Ramadan, El-Beih and Badr7). Preliminary trials have suggested possible hypoglycaemic, hypolipidaemic and immunomodulatory properties of the fenugreek plant(Reference Mowla, Alauddin and Rahman9, Reference Bin-Hafeez, Haque and Parvez11–Reference Belguith-Hadriche, Bouaziz and Jamoussi14). The present study aimed to evaluate and compare the efficacy of Egyptian fenugreek seed powder (FSP) in alleviating the experimentally induced metabolic syndrome (in two different animal models: type 1 diabetic and obese rat models) and immunosuppression as well as delay in burn-healing (for the first time, to our knowledge, in CP-treated rats). Two different doses of FSP (0·5 and 1·0 g/kg body weight) were used in the present study to test whether the obtained modulatory effects were dose dependent. Furthermore, the present study investigated any deleterious effects caused by consuming the Egyptian FSP.
Materials and methods
Materials
Alloxan monohydrate, cholesterol and CP (200mg/ampoule) were purchased from Sigma-Aldrich (St Louis, MO, USA), WinLab (Market Harborough, Leicestershire, UK) and Baxter Oncology GmbH (Frankfurt, Germany), respectively. Egyptian fenugreek (Trigonella foenum-graecum L. cultivar Baladi, family: Leguminosae, known locally as helba) seeds were purchased from a local herbal store (SMA Trading Company, Giza, Egypt), kindly authenticated by Professor Dr Raifa A. Hassanein (Botany Department, Faculty of Science, Ain Shams University, Cairo, Egypt), and a voucher specimen was deposited in our department. Adult male Wistar albino rats (Rattus norvegicus), weighing 125–135 g, were obtained from the National Research Centre in Giza, Egypt. Animals were housed in suitable cages and acclimatised to laboratory conditions for a period of 1 week before the commencement of the experiments. Rats were fed standard rodent food pellets (Agricultural–Industrial Integration Company, Giza, Egypt) and double-distilled water. The standard rodent food pellets contain wheat bran, dried clover, maize, bean hay, methionine, molasses, salt, in addition to mineral and vitamin mix. The amount of crude proteins, fats and fibres in the food pellets are 12, 2·4 and 14 %, respectively. The energy content of the standard diet is 920·48 kJ/100 g. All animals were humanely treated in accordance with the WHO guidelines for animal care, and the study design was approved by the Ain Shams University Research Ethics Committee.
Experimental design and treatment schedule
Experimental animals were randomly divided into twelve groups of five rats each: three healthy groups; three diabetic groups; three obese groups; three immunosuppressive groups. In the healthy groups, animals were treated by oral administration and daily with 0·5 or 1·0 g/kg body weight (low or high dose, respectively) of FSP suspended in 1·0 ml distilled water for 4 weeks. In the diabetic groups, animals were given the low or high dose of FSP orally and daily for 4 weeks and subcutaneously received an injection of alloxan (120 mg/kg body weight) for the first 3 d only to induce hyperglycaemia(Reference Ramadan, El-Beih and Abd El-Ghffar8). In the obese groups, animals were fed a hypercholesterolaemic diet (standard rodent food pellets plus 2 % cholesterol, w/w) to induce hyperlipidaemia(Reference Ramadan, El-Beih and Abd El-Ghffar8) and given the low or high dose of FSP orally and daily for 4 weeks. In the immunosuppressive groups, animals were given the low or high dose of FSP orally and daily for 4 weeks and received a single intraperitoneal injection (25 mg/kg body weight) of CP(Reference Ramadan, El-Beih and Badr7) on day 24 (i.e. 6 d before killing the animals) to induce immunosuppression. The healthy control, alloxan-only-treated, cholesterol-only-treated and CP-only-treated groups received 1·0 ml distilled water orally and daily, as a vehicle, instead of FSP suspension for 4 weeks.
Blood and tissue sampling
Animals were fasted overnight and subjected to light diethyl ether anaesthesia before killing on day 29. Blood was collected into clean test-tubes with or without EDTA. A portion of blood with EDTA (0·5 ml) was used to measure the total and differential leucocyte counts by Coulter (Hemat 8 analyser; SEAC, Freiburg, Germany). Another portion of blood without EDTA was used to separate serum, which was divided into samples and preserved at − 70°C for further analysis. Immediately after killing the animals, the thymus and spleen were separated out of the body, cleaned and weighed. Bone marrow was also collected from the left femur bone. The cellularity of lymphoid organs was measured by a Neubauer counting chamber (Paul Marienfeld GmbH, Bad Mergentheim, Germany) after lysing the erythrocytes by red blood erythrocyte lysis solution (AppliChem GmbH, Darmstadt, Germany). The liver was quickly perfused in situ (via the hepatic portal vein) with ice-cold saline (to remove erythrocytes and clots) and homogenised in cold buffer (0·1 m-phosphate buffer, pH 7·4). Then, the homogenate was stored at − 70°C until used for the determination of GSH through the reaction of its sulfhydryl group with Ellman's reagent to give 5-thio-2-nitrobenzoic acid(Reference Ellman15).
Measurements
Food intake (on a per-group basis) was measured weekly. Body-weight gain or loss was calculated by the following equation: body-weight gain or loss = body weight at the end of the experiment − body weight at the beginning of the experiment. Serum glucose concentration was colorimetrically estimated using glucose oxidase and peroxidase(Reference Trinder16). Serum total lipid concentration was chemically determined by the phosphovanillin method(Reference Knight, Anderson and Rawle17). Serum total cholesterol(Reference Allain, Poon and Chan18), TAG(Reference McGowan, Artiss and Strandbergh19) and HDL-cholesterol(Reference Grove20) concentrations were colorimetrically determined using peroxidase-coupled methods. Serum LDL-cholesterol concentration was calculated according to the equation of Friedewald et al. (Reference Friedewald, Levy and Fredrickson21): LDL-cholesterol = total cholesterol − (TAG/5) − HDL-cholesterol. Atherogenic indices were calculated as follows: atherogenic index (1) = total cholesterol:HDL-cholesterol ratio, atherogenic index (2) = LDL-cholesterol:HDL-cholesterol ratio. Serum alanine aminotransferase and aspartate aminotransferase (ASAT) activities were colorimetrically measured(Reference Reitman and Frankel22). Serum alkaline phosphatase (ALP) activity was estimated from the rate of conversion of p-nitrophenylphosphate to p-nitrophenol(Reference Wenger and Kaplan23). Serum γ-globulin concentration was estimated by the Helena cellulose acetate protein electrophoresis as described previously(Reference Ramadan, El-Beih and Badr7). The percentage of change of any parameter = ((T − C)/C) × 100, where T is the mean value of the parameter in the treated group and C is the mean value of the parameter in the control group.
Delayed type of hypersensitivity response
The delayed type of hypersensitivity response was determined as described previously(Reference Bin-Hafeez, Haque and Parvez11) with some modifications. Briefly, on day 29, animals were subcutaneously immunised with 2·25 × 1010 sheep red blood corpuscles (Institute of the Agricultural Researches, Giza, Egypt). On the fifth day of immunisation, the animals were again challenged with 1 × 1010 cells in the left hind footpad. The increase in footpad thickness was measured 24 h after the second challenge by vernier calliper (Samir and Ali Bookshop, Cairo, Egypt). The right footpad was injected with saline solution and served as the trauma control for non-specific swelling.
Skin-burning healing process
On day 29, hairs in the back of animals were shaved, and a skin burn was performed by a hot stamp under local dermatological anaesthetic spray (Lidocaine, The Arab Drug Company, Cairo, Egypt). Then, the healing process was followed as described previously(Reference Harding, Morris and Patel24), and photos were taken periodically.
Statistical analysis
Data are presented as means with their standard errors. Statistical analysis was performed with one-way ANOVA or the Friedman test (only for comparing the percentages of changes of all parameters), and the differences among groups were determined by Bonferroni's or Dunn's multiple comparison test, respectively(Reference Turner and Thayer25), using GraphPad Prism version 4.03 for Windows (GraphPad Software, Inc., San Diego, CA, USA). P values of < 0·05, < 0·01 and < 0·001 were considered statistically significant, highly significant and very highly significant, respectively.
Results
Anti-metabolic syndrome efficacy of fenugreek seeds in the type 1 diabetic rat model
The present study showed that treatment of rats with alloxan alone did not significantly alter (P>0·05, t = 2·761, difference between means − 0·0023, 95 % CI − 0·0049, 0·0003) the liver-weight:body-weight ratio, but induced a very highly significant increase (P < 0·001) in serum alanine aminotransferase (202 %), ASAT (279 %) and ALP (248 %) activities, glucose (226 %), total lipid (53 %), total cholesterol (46 %) and TAG (117 %) levels, and atherogenic indices (147–892 %) compared with the healthy control animals (Tables 1 and 2). On the other hand, it induced a very highly significant decrease (P < 0·001) in body weight (31 %) and liver GSH level (76 %) compared with the healthy control animals (Table 1). As shown in Tables 1 and 2, FSP significantly modulated all these harmful changes induced by alloxan (40–76 %, P < 0·05–0·001 compared with the alloxan-only-treated group). These modulatory effects significantly increased by increasing the dose of FSP in all cases (P < 0·001), except in the liver GSH and serum total cholesterol levels where no statistically significant difference in modulation (P>0·05) was found between both doses of FSP (Tables 1 and 2). Food intake was not significantly changed (P>0·05) in alloxan with/without FSP-treated groups compared with the healthy control group (data not shown).
Table 1 Modulatory effects of fenugreek seed powder (FSP) on body and liver weight, serum aminotransferases and alkaline phosphatase activities, and liver GSH level in the healthy, diabetic and obese rat models
(Mean values with their standard errors)

ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; ALP, alkaline phosphatase.
Mean values were significantly different from that of the control group: *P < 0·05, **P < 0·01, ***P < 0·001.
Mean values were significantly different from that of the alloxan-only-treated group: †P < 0·05, †††P < 0·001.
Mean values were significantly different from that of the cholesterol-only-treated group: ‡‡P < 0·01, ‡‡‡P < 0·001.
Table 2 Modulatory effects of fenugreek seed powder (FSP) on serum glucose, lipid profile and atherogenic indices in the healthy, diabetic and obese rat models
(Mean values with their standard errors)

Atherogenic index (1), total cholesterol:HDL-cholesterol ratio; atherogenic index (2), LDL-cholesterol:HDL-cholesterol ratio.
Mean values were significantly different from that of the control group: *P < 0·05, **P < 0·01, ***P < 0·001.
Mean values were significantly different from that of the alloxan-only-treated group: ††P < 0·01, †††P < 0·001.
Mean values were significantly different from that of the cholesterol-only-treated group: ‡‡‡P < 0·001.
The percentages of changes of all parameters measured in the alloxan-only-treated group and the groups treated with alloxan plus either the low or high dose of FSP were 154·9 (sem 70·7, P < 0·001), 93·1 (sem 49·3, P < 0·05) and 37·7 (sem 24·4, P>0·05), respectively, compared with the healthy control group. All of these results revealed that the most modulatory effects on diabetic rats were induced by the high dose of FSP.
Anti-metabolic syndrome efficacy of fenugreek seeds in the obese rat model
The present study showed that feeding rats a hypercholesterolaemic diet induced a very highly significant increase (P < 0·001) in the body weight (25 %), liver-weight:body-weight ratio (39 %), serum alanine aminotransferase (208 %), ASAT (223 %) and ALP (56 %) activities, glucose (63 %), total lipid (75 %), total cholesterol (139 %) and TAG (113 %) levels, and atherogenic indices (203–1554 %) compared with the healthy control animals (Tables 1 and 2). On the other hand, it induced a significant decrease (13 %, P < 0·05, t = 3·712, difference between means 1·234, 95 % CI 0·189, 2·279) in the liver GSH level compared with the healthy control animals (Table 1). As shown in Tables 1 and 2, FSP significantly modulated all these harmful changes induced by the hypercholesterolaemic diet (56–78 %, P < 0·01–0·001 compared with the cholesterol-only-treated animals), except that the low dose of FSP did not significantly change (P>0·05) the increase in the liver-weight:body-weight ratio and serum ALP activity shown in the cholesterol-treated animals. The modulatory effects significantly increased by increasing the dose of FSP in all cases (P < 0·01–0·001), except in the liver-weight:body-weight ratio, serum ALP activity, and liver GSH and serum total lipid levels where no statistically significant difference in modulation (P>0·05) was found between both doses of FSP (Tables 1 and 2). The liver GSH level was completely modulated in the group that received the hypercholesterolaemic diet plus the low dose of FSP (P>0·05, t = 1·041, difference between means − 0·346, 95 % CI − 1·391, 0·699); moreover, it significantly increased by 11 % above the normal level (P < 0·05, t = 3·531, difference between means − 1·174, 95 % CI − 2·219, − 0·129) in the group that received the hypercholesterolaemic diet plus the high dose of FSP, compared with the healthy control group (Table 1). Serum ASAT activity and glucose level became comparable with those of the healthy control group (P>0·05) in the group that received the hypercholesterolaemic diet plus the high dose of FSP only (Tables 1 and 2). Food intake was not significantly changed (P>0·05) in the cholesterol with/without FSP-treated groups compared with the healthy control group (data not shown).
The percentages of changes of all parameters measured in the cholesterol-only-treated group and the groups treated with cholesterol plus either the low or high dose of FSP were 213·6 (sem 113·6, P < 0·001), 93·9 (sem 44·2, P < 0·05) and 46·5 (sem 23·0, P>0·05), respectively, compared with the healthy control group. All of these results revealed that the most modulatory effects on obese rats were induced by the high dose of FSP. In addition, FSP was more efficient in alleviating the signs of the metabolic syndrome in the obese animals (over 9 %) than in the type 1 diabetic animals.
Immunostimulant efficacy of fenugreek seeds in the immunosuppressive rat model
The present study showed that treatment of rats with CP alone induced severe blood leucopenia (P < 0·001, t = 5·2, difference between means 2·4, 95 % CI 1·0, 3·8), which was mainly due to neutropenia (P < 0·001, t = 4·923, difference between means 1·483, 95 % CI 0·565, 2·401) and lymphopenia (P < 0·05, t = 3·422, difference between means 0·789, 95 % CI 0·087, 1·492), compared with the healthy control animals (Fig. 1). It also caused a significant decrease (P < 0·05–0·001) in weights (25–35 %) and cellularity (59–65 %) of lymphoid organs as well as serum γ-globulin level (51 %) and delayed type of hypersensitivity response (48 %) compared with the healthy control animals (Figs. 2 and 3). Moreover, it caused a delay in the skin-burning healing process by prolonging the inflammatory phase (swelling, redness and warmth) and the proliferative phase (mucus secretion and pellicle detachment), as shown on days 18 and 26 in the CP-only-treated animals v. days 8 and 18 in the healthy control animals, respectively (Fig. 4). The remodelling phase, the final phase where the intact skin replaced the burning one, the diameter of the burn decreased until complete recovery and the hairs grew again, started/ended on day 33/41 in the CP-only-treated animals v. day 26/33 in the healthy control animals, respectively. As shown in Figs. 1–4, both FSP doses completely modulated all immunosuppressive activities of CP (P>0·05 and P < 0·05–0·001 compared with the healthy control animals and CP-only-treated animals, respectively), except that the low dose of FSP partially modulated the decrease in the cellularity of lymphoid organs and serum γ-globulin level as well as the delay in the skin-burning healing process shown in the CP-treated animals (P < 0·05–0·001 compared with the healthy control animals). The immunosuppressive activity of CP decreased by 57–108 % in the FSP-treated animals. The modulatory effects on the spleen-weight:body-weight ratio and the cellularity of lymphoid organs significantly increased (P < 0·05 and < 0·001, respectively) by increasing the dose of FSP (Fig. 2).

Fig. 1 Modulatory effects of fenugreek seed powder (FSP) on the changes in blood (a) total and (b) differential leucocyte counts in the healthy and immunosuppressed rat models. Values are means, with standard errors represented by vertical bars. Mean values were significantly different from that of the control group: *P < 0·05, ***P < 0·001. Mean values were significantly different from that of the cyclophosphamide (CP)-only-treated group: †P < 0·05, ††P < 0·01, †††P < 0·001. (a) ![]() , Total leucocytes;
, Total leucocytes; ![]() , total granulocytes; □, total agranulocytes. (b)
, total granulocytes; □, total agranulocytes. (b) ![]() , Basophils;
, Basophils; ![]() , eosinophils;
, eosinophils; ![]() , neutrophils; □, monocytes;
, neutrophils; □, monocytes; ![]() , lymphocytes.
, lymphocytes.
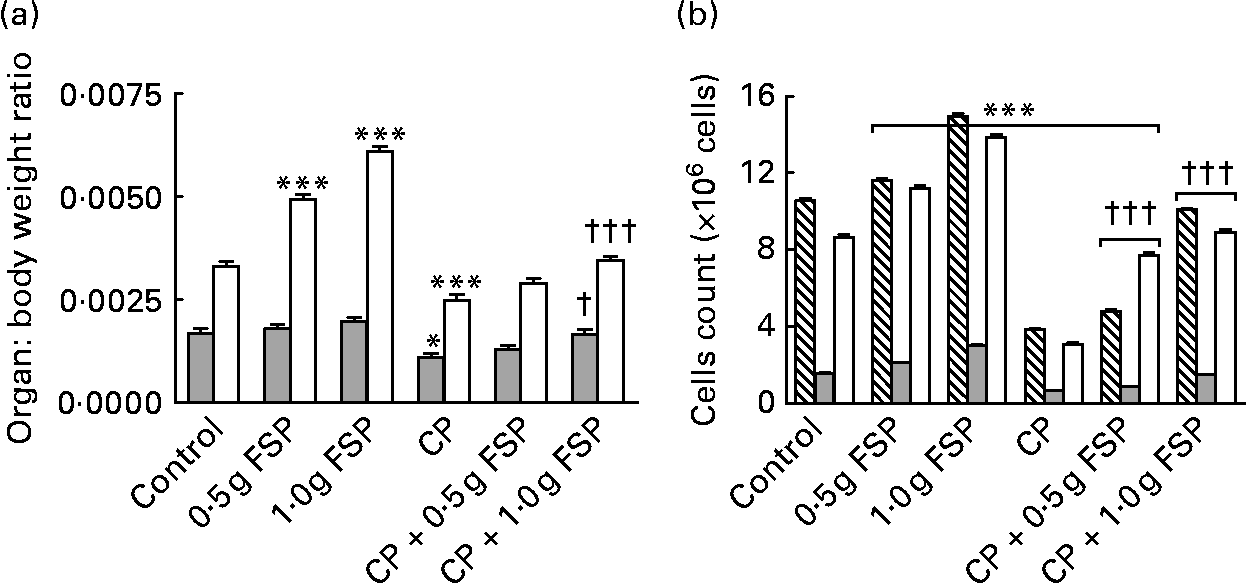
Fig. 2 Modulatory effects of fenugreek seed powder (FSP) on the changes in weights (a) and cellularity (b) of lymphoid organs in the healthy and immunosuppressed rat models. Values are means, with standard errors represented by vertical bars. Mean values were significantly different from that of the control group: *P < 0·05, ***P < 0·001. Mean values were significantly different from that of the cyclophosphamide (CP)-only-treated group: †P < 0·05, †††P < 0·001. (a) ![]() , Thymus; □, spleen. (b) ▧, Bone marrow;
, Thymus; □, spleen. (b) ▧, Bone marrow; ![]() , thymus; □, spleen.
, thymus; □, spleen.
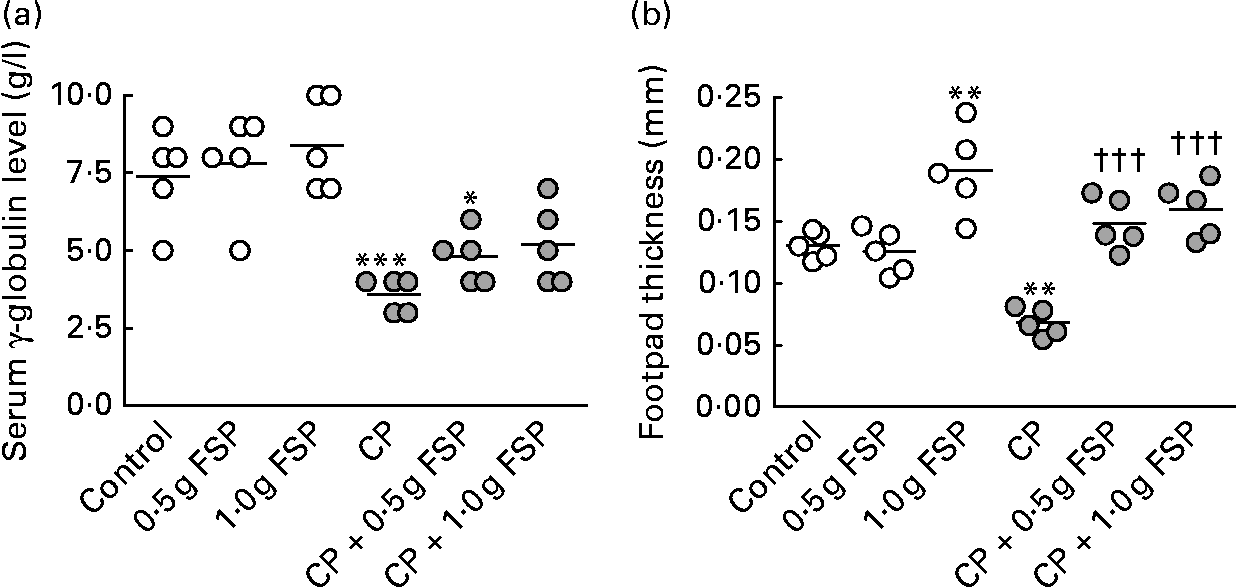
Fig. 3 Modulatory effects of fenugreek seed powder (FSP) on the serum γ-globulin level (a) and delayed type of hypersensitivity response (b) in the healthy and immunosuppressed rat models. Individual values are shown, with means represented by horizontal bars. Mean values were significantly different from that of the control group: *P < 0·05, **P < 0·01, ***P < 0·001. Mean values were significantly different from that of the cyclophosphamide (CP)-only-treated group: †††P < 0·001.

Fig. 4 Modulatory effects of fenugreek seed powder (FSP) on the skin-burning healing process in the healthy and immunosuppressed rat models. Photos were taken at 1, 8, 18, 26 and 33 d after burning the skin (a–e, respectively). CP, cyclophosphamide. (A colour version of this figure can be found online at journals.cambridge.org/bjn).
The percentages of changes of all parameters measured in the CP-only-treated group and the groups treated with CP plus either the low or high dose of FSP were − 38·6 (sem 5·0, P < 0·001), − 16·7 (sem 5·5, P>0·05) and 2·9 (sem 6·0, P>0·05), respectively, compared with the healthy control group. All of these results revealed that the most modulatory effects on immunosuppressive rats were induced by the high dose of FSP. In addition, the modulatory effects of FSP on CP-induced immunosuppression exceeded that on alloxan-induced type 1 diabetes and cholesterol-induced obesity (24 and 15 %, respectively).
Beneficial and deleterious effects caused by fenugreek seed consumption in healthy rats
The present study showed that treatment of healthy rats with either the low or high dose of FSP alone significantly increased (P < 0·001) the liver GSH level (19–32 %; Table 1), the spleen-weight:body-weight ratio (49–84 %; Fig. 2(a)) and the cellularity of lymphoid organs (10–91 %; Fig. 2(b)), accelerated the skin-burning healing process (Fig. 4) and significantly decreased (P < 0·01–0·001) the serum TAG level (15–34 %; Table 2) compared with the healthy control animals. These beneficial effects significantly increased by increasing the dose of FSP in all cases (P < 0·01–0·001). On the other hand, only the high dose of FSP significantly decreased (P < 0·05–0·001) the serum glucose (19 %), total lipid (22 %) and total cholesterol (11 %) levels as well as the first atherogenic index (17 %), and significantly increased (47 %, P < 0·01, t = 4·492, difference between means − 0·061, 95 % CI − 0·102, − 0·020) the delayed type of hypersensitivity response in the healthy rats compared with the healthy control animals (Table 2 and Fig. 3). All other parameters measured in the present study did not significantly alter in the healthy rats by both FSP doses (P>0·05 compared with the healthy control animals). Moreover, food intake, serum total protein, albumin, globulin fractions (α1-, α2-, β- and γ-globulins) and bilirubin (total, direct and indirect) levels, erythrocyte and platelet counts, Hb content, packed cell volume and blood indices were not significantly changed (P>0·05) in the healthy rats that received either the low or high dose of FSP compared with the healthy control animals (data not shown). The only deleterious effect detected in the present study was the significant increase (18 %, P < 0·05) in the clotting time in rats that received the high dose of FSP only compared with the healthy control animals (data not shown). All of these results indicated that FSP doses used in the present study were considered safe in general.
Discussion
Rats treated with alloxan (a pancreatic β-cell cytotoxin) and rats fed a hypercholesterolaemic diet display most signs of the metabolic syndrome including hyperglycaemia and dyslipidaemia, which mimic those developed in human hypoinsulinaemia or type 1 diabetes and insulin resistance or type 2 diabetes, respectively(Reference Ramadan, El-Beih and Abd El-Ghffar8). The present study showed that FSP significantly alleviated (P < 0·01–0·001) most signs of the metabolic syndrome including hyperglycaemia, hyperlipidaemia (hypercholesterolaemia and hypertriacylglycerolaemia), elevation in atherogenic indices and impairment of liver functions resulting from experimentally induced type 1 diabetes and obesity in male albino rats by alloxan and a hypercholesterolaemic diet, respectively (Tables 1 and 2). These beneficial effects of FSP were dose dependent in most cases. The hypolipidaemic effect and the marked decrease in atherogenic indices shown in the present study by FSP in rats treated with alloxan or fed a hypercholesterolaemic diet (Table 2) may reduce the incidence of atherosclerosis in diabetic and obese patients.
Fenugreek seeds are used as a condiment, as a supplement to wheat and maize flour for bread making and as a constituent of the daily diet of the general population in many countries(Reference Bin-Hafeez, Haque and Parvez11). They contain 58 % carbohydrates (mainly a water-soluble galactomannan), 23–26 % protein, 6–7 % fat (mainly PUFA), 5–6 % saponins and 2–3 % alkaloids such as trigonelline(Reference Belguith-Hadriche, Bouaziz and Jamoussi14, Reference Srichamroen, Thomson and Field26). Soluble dietary fibres of fenugreek seeds delay carbohydrate digestion and absorption by slowing gastric emptying, inhibiting the activity of intestinal disaccharidases and brush-border membrane glucose carriers, increasing gastrointestinal motility and enhancing insulin action(Reference Hannan, Ali and Rokeya13, Reference Srichamroen, Thomson and Field26, Reference Mathern, Raatz and Thomas27). In addition, the alcoholic extract of fenugreek seeds was found to suppress glucose absorption and hepatic gluconeogenesis by inhibiting the activity of intestinal brush-border α-amylase (that digests starch and dextrin) and glucose-6-phosphatase, respectively, and to increase glucose metabolism by increasing the activity of glucose-6-phosphate dehydrogenase, a hexose monophosphate pathway shunt enzyme(Reference Gad, El-Sawalhi and Ismail28). Moreover, the amino acid, 4-hydroxyisoleucine, of fenugreek seed has insulin secretagogue activity(Reference Sauvaire, Petit and Broca29). Diosgenin (a major aglycone of saponins) improves glucose metabolism and dyslipidaemia in obesity-related diabetes by inhibiting inflammation in adipose tissues and decreasing the size of adipocytes, which are inversely related to insulin sensitivity, through stimulating adiponectin expression in adipose tissues and promoting adipocyte differentiation, respectively(Reference Uemura, Hirai and Mizoguchi30). In addition, sapogenins, such as diosgenin, show lipid-lowering activity by increasing biliary cholesterol excretion(Reference Basch, Ulbricht and Kuo31). Fenugreek seeds are also rich in flavonoids (>100 mg/g), such as quercetin and naringenin(Reference Belguith-Hadriche, Bouaziz and Jamoussi14). Quercetin prolongs insulin signalling by improving tyrosine phosphorylation through the activation and inhibition of protein tyrosine kinases and protein tyrosine phosphatases, respectively(Reference Kannappan and Anuradha32). Naringenin inhibits 3-hydroxy-3-methylglutaryl-CoA reductase and cholesterol acyltransferase, a rate-limiting enzyme in cholesterol biosynthesis and a key enzyme involved in the esterification and absorption of cholesterol, secretion of hepatic LDL-cholesterol, and cholesterol accumulation in the arterial wall, respectively(Reference Belguith-Hadriche, Bouaziz and Jamoussi14). All of the aforementioned reports may explain the anti-metabolic syndrome activity of FSP shown in the present study.
An interesting finding in the present study was that FSP significantly modulated (P < 0·001) both the severe decrease (31 %) and increase (25 %) in body weight induced by alloxan and a hypercholesterolaemic diet, respectively (Table 1), without changing food consumption (data not shown). These results suggest that FSP may have a positive anabolic effect in the type 1 diabetic rats by decreasing the degeneration of the adipocytes and muscle tissues through improving glucose metabolism. Moreover, the significant decrease (P < 0·001) in body-weight gain in addition to the liver-weight:body-weight ratio shown in the obese rats that received the high dose of FSP may be due to a reduction in body and liver fats by improving lipid metabolism, increasing energy expenditure and affecting fat absorption and excretion. Thus, the results of the present study suggest a direct effect of FSP on the improvement of metabolism rather than affecting food intake.
Recently, attention has been focused on the relationship between production of free radicals, especially reactive oxygen species, and the pathogenesis and progression of many diseases including diabetes mellitus. Mechanisms that contribute to the formation of free radicals in diabetes mellitus may include metabolic stress resulting from changes in energy metabolism, inflammatory mediators and impaired antioxidant defence mechanisms(Reference Anwar and Meki33). There is a strong positive correlation between the production of reactive oxygen species in addition to a decrease in endogenous radical scavengers and hyperglycaemia, dyslipidaemia, membrane lipid peroxidation and cellular injury caused by alloxan and a hypercholesterolaemic diet(Reference Belguith-Hadriche, Bouaziz and Jamoussi14, Reference Szkudelski34–Reference Czako, Szabolcs and Vajda36). GSH, the first-line defence against lipid peroxidation, is an essential electron donor to glutathione peroxidases in the reduction of hydroperoxides and serves as a nucleophilic co-substrate to glutathione-S-transferases in the detoxification of xenobiotics(Reference Barber and Harris37). The reduction of cellular toxicity markers (serum alanine aminotransferase, ASAT and ALP activities) and the elevation in the liver GSH level shown in the type 1 diabetic and obese rats by consuming FSP (Table 1) indicated that the chemical components of FSP prevented hepatocellular damage by stabilising the integrity of the cell membrane, keeping the membrane intact and the enzymes enclosed, through scavenging free radicals. This antioxidant activity of fenugreek seeds may be attributed to the active H-donating ability of the hydroxyl substitutions of their flavonoids(Reference Kaviarasan, Vijayalakshmi and Anuradha38). The modulatory effect of FSP on the liver GSH level was either partial or complete in the type 1 diabetic or obese rat model, respectively. Moreover, the liver GSH level in the obese rats that received the high dose of FSP exceeded that in the healthy control animals. These results may explain why FSP was more efficient in alleviating the signs of the metabolic syndrome in the obese animals (over 9 %) than in the type 1 diabetic animals.
CP is a commonly used anti-cancer drug that causes toxicity by its reactive metabolites such as acrolein and phosphoramide mustard, which induce oxygen radical formation and immunosuppression, respectively(Reference Bhatia, Kaur and Atif39). The present study showed, probably for the first time, that FSP (especially the high dose) completely modulated the immunosuppressive activity of CP including leucopenia (which was mainly due to neutropenia and lymphopenia), the decrease in weights and cellularity of lymphoid organs, the deficiency in humoral and cellular immune responses (as indicated by the decrease in the serum γ-globulin level and delayed type of hypersensitivity response, respectively), and the delay in the skin-burning healing process (Figs. 1–4). Therefore, FSP may reduce the incidence of infections in cancer patients treated with such chemotherapeutic agents. The significant increase (P < 0·001) induced by FSP in bone marrow cellularity in the CP-treated rats (Fig. 2(b)) indicated a stimulatory effect of fenugreek seeds on bone marrow haematopoietic stem cells, which may be, in part, due to the considerable amount of Fe found in fenugreek seeds(Reference Bin-Hafeez, Haque and Parvez11). The increase in the weight of lymphoid organs (thymus and spleen) induced by FSP in the CP-treated animals was concomitant with the increase in their cellularity (Fig. 2). Another study found that 100 mg/kg body weight of an Indian fenugreek aqueous extract was the optimum dose that induced a stimulatory effect on immune functions in healthy mice, and this immunostimulant activity of fenugreek decreased by increasing the dose(Reference Bin-Hafeez, Haque and Parvez11). In the present study, the immunostimulant activity of Egyptian fenugreek seeds increased by increasing the dose from 0·5 to 1·0 g/kg body weight in immunosuppressed rats. The immunostimulant activity of fenugreek seeds may be due to the mitogenic and antioxidant properties of its constituents, which stimulate the immunocompetent cells(Reference Bin-Hafeez, Haque and Parvez11, Reference Devasagayam and Sainis40). Saponins, fibres and flavonoids of fenugreek seeds are the most likely components responsible for their immunostimulant activity(Reference Bin-Hafeez, Haque and Parvez11).
There is an overall decrease in the glutathione content in various tissues as a result of CP treatment(Reference Bhatia, Kaur and Atif39). Acrolein induces the GSH depletion by interacting with its amino acid, cysteine(Reference Kehrer and Biswal41). Restoration of the GSH level by FSP, as shown in the present study in the type 1 diabetic and obese rats, may play an important role in reversing CP-induced immunosuppression. CP is known to result in hypercholesterolaemia and hypertriacylglycerolaemia, which are well-known risk factors in CVD(Reference Mythili, Sudharsan and Sudhahar6). The hypolipidaemic activity of FSP shown in the present study may protect against hyperlipidaemic cardiomyopathy in CP-treated cancer patients. Another interesting finding in the present study was that the modulatory effects of FSP on CP-induced immunosuppression exceeded that on alloxan-induced type 1 diabetes and cholesterol-induced obesity by 24 and 15 %, respectively. This result indicated that the immunostimulant activity of fenugreek seeds exceeded their anti-metabolic syndrome activity. The FSP doses used in the present study were considered safe in general, despite the mild increase in the clotting time shown in rats that received the high dose of FSP (data not shown), which may be due to the coumarin derivatives existing in fenugreek seeds(Reference Basch, Ulbricht and Kuo31). In conclusion, fenugreek seeds may be useful not only as a dietary adjunct for the control of the metabolic syndrome in diabetic and obese patients, but also as an immunostimulant in immunocompromised patients such as those under chemotherapeutic interventions.
Acknowledgements
The present study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. N. M. E.-B. and G. R. planned the study, designed all the experiments, summarised, discussed and interpreted the results, and drafted the manuscript. H. F. A. E.-K. carried out the experiments and performed the statistical analysis with assistance from G. R. The authors have no potential financial conflict of interest.










