Introduction
Protein digestibility, an indirect measure of the extent of the digestion of protein into amino acids and their subsequent absorption, is a key determinant of protein bioavailability(Reference Fuller and Tomé1) and is thus an important factor for nutritional quality assessment(2). Differences in digestibility exist among protein sources, especially between those used in diets from developed and developing countries. Values for true faecal nitrogen digestibility of 88–94 % have been reported(Reference Gilani, Cockell and Sepehr3) for typical North American diets compared to values of 54–78 % for diets found in developing countries (India, Guatemala or Brazil and based on less-refined cereals and grain legumes). Within protein sources, differences also exist among amino acids; for instance, the true ileal digestibility of lysine from bovine casein (97·4 %) being considerably higher than that of serine (87·0 %)(Reference Deglaire, Bos and Tomé4). This highlights the importance of determining both nitrogen and amino acid digestibility with accuracy. It is technically, economically and ethically difficult to determine protein digestibility in humans. This is particularly so for ileal digestibility, which can only be determined in humans that have either previously undergone an ileostomy operation(Reference Moughan, Butts and van Wijk5) or that are equipped with a naso-ileal tube(Reference Mahé, Huneau and Marteau6). Neither of these methods is suitable for routine application. Thus, animal models have been extensively used, with rats and pigs being the most commonly employed(Reference Fuller and Tomé1).
The accuracy of the protein digestibility measure can be improved after correction for endogenous protein losses, thus providing a measure of true (or standardised) protein digestibility(Reference Stein, Sève and Fuller7). Such a digestibility value reflects the specific fate of dietary nitrogen within the gut and allows the metabolic costs associated with synthesis and the recycling of gut endogenous amino acid losses to be represented(Reference Stein, Sève and Fuller7). A further improvement in accuracy can be obtained by determining digestibility at the ileal rather than at the faecal level(Reference Fuller and Tomé1, Reference Darragh and Hodgkinson8–Reference Moughan9). The high metabolic activity of the hindgut microflora likely modifies the undigested dietary amino acid profile; thus leading to some degree of overestimation or, in some cases, of underestimation of digestibility. Additionally, amino acids as such, are mostly absorbed in the small intestine(Reference Krawielitzki, Zebrowska and Schadereit10–Reference Fuller and Reeds11), but it remains unclear whether the colon may also absorb amino acids to a limited extent(Reference Blachier, Mariotti and Huneau12). Overall, true ileal nitrogen (and amino acid) digestibility should be a better predictor of bioavailability than digestibility measured at the faecal level(Reference Fuller and Tomé1, Reference Hodgkinson, Souffrant and Moughan13). Nevertheless, true faecal nitrogen digestibility is of interest for assessing whole-body nitrogen losses(Reference Fuller and Tomé1) and this value, as determined in the growing rat, is currently used for calculating the recommended dietary protein quality index, the protein digestibility-corrected amino acid score or PDCAAS(2).
The growing rat has been recommended and is generally accepted as a valid animal model for predicting protein digestibility in humans(14); however, the pig has also been promoted as a useful model for human nutrition studies(Reference Deglaire, Bos and Tomé4, Reference Darragh and Hodgkinson8, Reference Moughan, Cranwell and Darragh15–Reference Moughan16). The aim here was to evaluate and compare the suitability of these species as animal models for assessing ileal and faecal amino acid and nitrogen digestibility in humans. Studies providing nitrogen and amino acid digestibility data for the growing rat or for the growing pig in comparison with comparable data from the adult human were reviewed. Drawbacks and advantages of both species are discussed.
The growing rat and adult human
Faecal digestibility
The faecal digestibility of dietary protein from animal, vegetable and mixed sources has been directly compared between humans and rats in previous studies(Reference Bach Knudsen, Wisker and Daniel17–Reference Wisker, Bach Knudsen and Daniel20), as reported in Table 1. The rat coefficients of apparent faecal nitrogen digestibility overestimated on average by 13 % the human coefficients and the rat digestibility values were not significantly correlated (r = − 0·14, P = 0·75) with the human coefficients (Table 1). In the review of Ritchey & Taper(Reference Ritchey and Taper21), the difference between human and rat coefficients of apparent faecal nitrogen digestibility was reported to be slightly lower, with rat values being on average 6 % higher than the human ones. The degree of correlation between the two species over seven different protein sources (r = 0·17, P = 0·73) was low and statistically non-significant. When true faecal nitrogen digestibility was determined (Table 1), a better agreement between the species was found, with most of the differences being statistically non-significant and with the rat values being on average only 3 % higher than the human ones and with a high and significant correlation between the species (r = 0·92, P = 0·001). The better agreement between species for true digestibility is due to the correction for endogenous nitrogen losses, which appears to contribute to total faecal nitrogen losses in a greater manner in humans than in rats. Although not direct evidence, this observation is supported by a reported higher ileal endogenous nitrogen flow in humans than in rats (Table 2). The large intestine of the rat is anatomically different to that of the adult human. The rat does not have a sigmoid colon but has a larger caecum relative to the overall size of its digestive tract in comparison with man(Reference DeSesso and Jacobson42). Such differences in the large intestine possibly support different microbial modifications of undigested and unabsorbed nitrogenous compounds. Therefore, true ileal nitrogen and amino acid digestibility, which is likely a more accurate and sensitive measure(Reference Fuller and Reeds11), may be more comparable between the species.
Table 1 Apparent and true faecal digestibility of nitrogen as determined in adult humans and growing rats
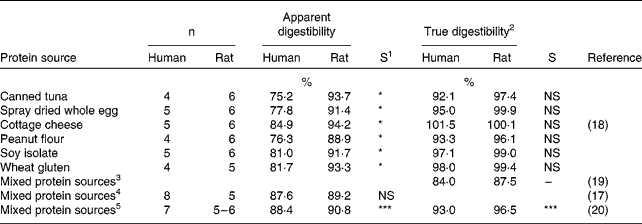
1 Statistical significance as reported in the studies: *, P < 0·05; ***, P < 0·001; NS, non significant.
2 Corrected using a constant estimate of faecal endogenous nitrogen loss.
3 Mean digestibility over 2 mixed diets based on vegetable or vegetable/animal proteins.
4 Vegetable and animal proteins. Mean digestibility over 4 diets with similar protein content but different fibre contents. For each diet, there was no statistically significant difference between species.
5 Vegetable and animal proteins. Mean over 3 diets with similar protein content but different fibre contents. For each diet, there was a significant difference (P < 0·05) between species.
Table 2 Ileal endogenous nitrogen flow and its amino acid composition (mean ± SD) determined in the rat, pig and human fed a protein-free diet

1 Nitrogen flow(Reference Butts, Moughan and Smith22–Reference Moughan, Buttery and Essex27). Amino acid composition(Reference Butts, Moughan and Smith22–Reference Donkoh, Moughan and Morel24).
3 References(Reference Fuller, Milne and Harris37–Reference Rowan, Moughan and Wilson39). Values from references (37,39) were calculated for an average daily dry matter intake of 410 g determined according to the data from Department for Environment(40) and Marriott & Buttriss(Reference Marriott and Buttriss41).
Ileal digestibility
In the work of Deglaire(Reference Deglaire43), true ileal amino acid and nitrogen digestibility were compared directly between humans and rats (Table 3). Similar methods to determine endogenous protein losses (isotope dilution) were employed in both species. Ileal digesta were sampled via naso-ileal intubation in humans, whereas ileal digesta were collected in rats after euthanasia. Amino acid digestibility coefficients were highly correlated between humans and rats, and the correlation coefficient was higher for true ileal digestibility (r = 0·91, P < 0·001) than for apparent ileal digestibility (0·83, P ≤ 0·001). There were statistically significant differences in digestibility between the human and rat, but the differences were lower for true digestibility (5 % on average) than for apparent digestibility (10 % on average). Ileal nitrogen digestibility was much closer between species for the true digestibility coefficients, being either similar for casein or only 4 % higher for rats given hydrolysed casein. Apparent coefficients of nitrogen digestibility were on average 18 % higher in rats for intact and hydrolysed casein. To our knowledge, the work conducted by Deglaire(Reference Deglaire43) is the only controlled study directly comparing ileal protein digestibility in rats and humans. Overall, there was generally close agreement between the two species for true ileal nitrogen and amino acid digestibility in the two highly digestible proteins, but a much wider range of protein sources needs to be examined, before definitive conclusions can be made.
Table 3 Apparent and true ileal amino acid and nitrogen digestibility coefficients for meals based on intact casein or on hydrolysed casein fed to growing rats and adult humans1
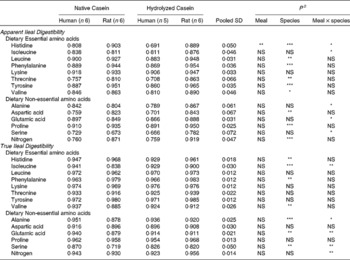
1 Data from reference(Reference Deglaire43).
2 Statistical significance as reported in the study: *, P < 0·05; ***, P < 0·001; NS, non significant.
Based on true ileal amino acid digestibility values obtained from indirect comparisons, Fuller & Tomé(Reference Fuller and Tomé1), observed that human and rat values were in good general agreement for plant (soya) and animal (milk) proteins with only small differences between digestibility coefficients. The exception was for glycine digestibility, which was on average 7 % lower in rats fed casein or soya protein isolate(Reference Fuller and Tomé1). This difference may be due to the different methods used to determine endogenous losses; the isotope dilution method in humans(Reference Gaudichon, Bos and Morens44) and the enzyme-hydrolysed protein/ultrafiltration method in rats(Reference Rutherfurd and Moughan45). The latter method is known to underestimate endogenous glycine flow(Reference Hendriks, Sritharan and Hodgkinson25, Reference Rutherfurd and Moughan45–Reference Moughan, Schuttert and Leenaars46). Other indirect studies have reported similar inter-species true ileal nitrogen digestibility values for wheat, with values of 90 % in humans(Reference Bos, Juillet and Fouillet47) and 91 % in rats(Reference Eggum, Hansen and Larsen48–Reference Sarwar, Peace and Botting49). However, recent data suggest that rats may be better able to digest some proteins that are poorly digestible in humans, such as rapeseed protein, for which true ileal nitrogen digestibility was 84–87 % in humans versus 95 % in rats(Reference Bos, Airinei and Mariotti50–Reference Boutry, Chevalier and Fouillet51). A similar high digestibility of 93–95 % for rapeseed protein has been previously reported in rats(Reference Sarwar52). The low digestibility in humans may be due to the presence of some protein fractions particularly resistant to hydrolysis by human pepsin(Reference Bos, Airinei and Mariotti50). In pigs fed the same rapeseed isolate, an intermediate true ileal digestibility value of 91 % was found(Reference Deglaire, Bos and Tomé4). Further controlled comparisons should be undertaken between rats and humans, especially for poorly digestible proteins (e.g. lentil, kidney bean, pinto bean).
As observed at the faecal level, there was a greater difference between rats and humans for apparent ileal digestibility than for true ileal digestibility. This likely results from a higher contribution of endogenous nitrogen losses to total nitrogen losses in humans than in rats. Based on previous results, endogenous nitrogen flow (μg/g dry matter intake) appears to be 45 % higher in humans than in rats (Table 2). Nevertheless, it is noteworthy that the amino acid compositions of their endogenous protein losses are relatively similar (Table 2), except for the glutamic acid concentration which appears to be three times higher in rats than in humans.
Overall, there seems to be reasonably close agreement between the growing rat and adult human for true ileal amino acid and nitrogen digestibility. This supports the widely held belief that rats and humans digest a variety of food proteins to a similar extent(Reference Gilani, Cockell and Sepehr3).
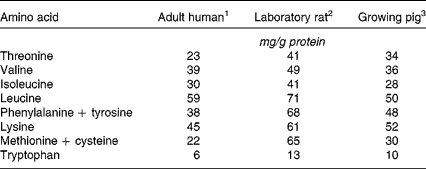
In spite of this, the relevance to humans of rat digestive and metabolic data must be interpreted with some caution, due to pronounced nutritional, physiological and anatomical differences between the two species. A major inter-species discrepancy is found in amino acid requirements, as shown by amino acid scoring patterns (Table 4). The human/rat difference is particularly dramatic for the sulphur-containing amino acids, for which the rat amino acid scoring value is more than double the human one. This is one of the reasons why dietary protein quality indices for humans are no longer based on protein efficiency ratio or net protein ratio, as determined in the rat, but rather on PDCAAS, which relies only on true faecal nitrogen digestibility, as determined in the rat.
Table 4 Amino acid scoring pattern (amino acid requirements expressed relative to the recommended protein intake) across species
Adult humans have higher maintenance amino acid requirements than growing rats(Reference Ritchey and Taper21). Nevertheless, Mitchell(Reference Mitchell55), who compared determined biological values of proteins, reported a better correlation between the growing rat and adult man (r = 0·92) than between the adult rat and adult man (r = 0·67). The upper digestive tract (mouth to ileum) is similar between rats and humans both anatomically and physiologically(Reference Kararli56). In particular, the transit rate of digesta is comparable between these species, with food traversing the entire small intestine in approximately 3 to 4 h in both rats and humans(Reference DeSesso and Jacobson42). However, there are major differences between rats and humans in the size and architecture of the hindgut(Reference Ritchey and Taper21, Reference Kararli56). Furthermore, coprophagy occurs in the rat, which might impair protein quality assessment if not prevented. Finally, the rate of small intestinal mucosa protein synthesis appears to be substantially higher in rats than in humans. The average fractional protein synthesis rate (g protein synthesized/100 g protein in the tissues) was 143 % per day for rats and ranged from 22 to 50 % in humans, as reviewed by Waterlow(Reference Waterlow57). This suggests a higher mucosal protein renewal, possibly leading to a higher degree of dietary nitrogen recycling within endogenous mucosal protein. This is supported by results showing that 65 % of the dietary nitrogen collected at the ileal level in rats had been recycled within endogenous protein ( ≥ 10-kDa)(Reference Deglaire, Moughan and Bos58), whereas in humans this amounted to 11 % only(Reference Deglaire43). In the latter studies, however, dietary nitrogen recycling may have been maximized by the experimental design used for the rats (i.e. digesta sampling after 8 h of continuous feeding) unlike that for humans receiving a bolus meal 8 hours before sampling. Dietary nitrogen recycling within endogenous protein may lead to some underestimation of true nitrogen digestibility, when the latter is determined using the isotope dilution method. Whereas the impact appears to be small in humans this underestimation was greater in rats for a diet based on hydrolysed casein.
Further important differences relate to behaviour, with the rat being nocturnal and having a nibbling eating habit(Reference Cohn and Joseph59). It is also noteworthy that the dental anatomy of the rat is different from that of the human with the rat lacking canines and premolars.
Overall and taking into consideration both potential anatomical and physiological differences in digestion between rats and humans and the results of direct comparisons between the species for protein and amino acid digestibility, it is concluded that the rat is a useful model for humans for determining protein digestibility(Reference Fuller and Tomé1, Reference Bergen60). In particular, the growing rat offers logistical advantages. It is readily available, easily housed and cared for, and is an economical option, especially compared with the pig(Reference Coles, Moughan and Awati61), in particular in terms of purchase price, space and food requirements and care and maintenance requirements.
The growing pig and adult human
With a gastrointestinal system that is very similar anatomically and physiologically to that of the adult human, the growing pig has been used extensively for studying aspects of human nutrition(Reference Bergen60, Reference Guilloteau, Zabielski and Hammon62), including aspects of protein digestion and metabolism.
Faecal digestibility
Darragh & Moughan(Reference Darragh and Moughan63) reported good agreement for apparent faecal protein digestibility between piglets and infants fed a milk formula. This was especially the case for the amino acids, for which apparent digestibility did not differ significantly (P>0·05) between species, except for threonine, serine, glutamic acid and proline. On average, apparent amino acid digestibility was only 1 % higher in piglets than in infants. Similarly, apparent faecal nitrogen digestibility was only slightly higher in piglets than in infants, with values of 97·5 and 94·5 %, respectively. This supported the earlier observations of Forsum et al. (Reference Forsum, Goranzon and Rundgren19), in which the true faecal nitrogen digestibilities of diets based on mixed proteins (mainly cereals with plant proteins such as beans, peas and soya or with animal proteins such as meat and fish) were found to be slightly higher (4 % on average) in growing pigs than in adult humans. It is noteworthy that the nitrogen digestibility of the diet containing animal protein was closer between pigs and humans (+2 %) than that of the diet containing only plant protein (+4 %). Overall, these two controlled studies suggest close agreement between faecal amino acid and nitrogen digestibility in pigs and humans.
Ileal digestibility
Few studies have directly compared ileal protein digestibility between pigs and humans. A controlled study was undertaken by Rowan et al. (Reference Rowan, Moughan and Wilson39), who used the same method to determine digestibility in both species. Ileal protein digestibility of a typical Western diet (with mixed protein sources) was determined in ileostomized human subjects and ileostomized pigs, with true digestibility being determined after correction for endogenous losses (protein-free diet). Values for apparent ileal digestibility differed significantly (P < 0·05) between species for half of the determined amino acids. However, in these cases, digestibility in the pig was on average only 3 % higher than in humans. For true ileal nitrogen and amino acid digestibility, there were no statistically significant differences found between species, except for the amino acids phenylalanine, threonine, methionine and cysteine for which digestibility was significantly lower (5 %) in pigs than in humans. This inter-species closeness for true ileal protein digestibility is supported by a recent controlled digestibility study(Reference Deglaire, Bos and Tomé4) of plant (rapeseed) and animal proteins (native intact and hydrolysed casein) in healthy growing pigs and healthy adult humans, whereby ileal digesta were collected through a post-valve T-caecum cannula in pigs and through a naso-ileal tube in adults(Reference Deglaire, Bos and Tomé4). Endogenous nitrogen and amino acid losses were determined by isotope (15N) dilution in both species. Whereas apparent ileal digestibility was found to be statistically significantly higher in pigs than in humans for all amino acids (9 % higher in pigs) and for nitrogen (17 % higher in pigs), the differences for true digestibility were small, being statistically significantly higher in the pig only for phenylalanine, tyrosine, lysine, histidine, aspartic acid and for nitrogen. Where statistically significant differences were found, true ileal digestibility was on average 3 % higher in pigs than in humans. Both apparent and true ileal amino acid digestibility were highly correlated between species (r = 0·89, P < 0·001). Combining the data for both controlled studies(Reference Deglaire, Bos and Tomé4, Reference Rowan, Moughan and Wilson39), the rankings of the protein sources (mixed diet, rapeseed, intact casein, hydrolysed casein) for digestibility were similar between species, and there was a high degree of correlation (r = 0·94), which was close to statistical significance (P = 0·06)(Reference Deglaire, Bos and Tomé4). Overall, these studies show close agreement between pigs and humans, especially for true ileal digestibility, for which differences between species did not exceed 5 % and were limited to only a few of the amino acids.
Indirect comparisons also show agreement between pigs and humans for the true ileal amino acid digestibility of plant and animal proteins (soya protein and casein)(Reference Fuller and Tomé1). Amino acid digestibilities were on average only 4 % higher in pigs than in humans, except for the amino acids phenylalanine, glycine and cysteine for which digestibility was on average 5 % lower in pigs than in humans. For wheat protein, true ileal nitrogen digestibility when determined by the isotope dilution method, was in the same range for humans (90 %)(Reference Bos, Juillet and Fouillet47) and for pigs (93 %)(Reference de Vrese, Frik and Roos64). A similar digestibility value to that found in humans for wheat was also found in pigs (89 %), when endogenous losses were determined by the multiple regression analysis method(Reference Jondreville, van den Broecke and Gatel65). Similarly, true ileal nitrogen digestibility of native casein, determined by the isotope dilution method, was in the same range in humans, with values of 94–95 %(Reference Deglaire, Bos and Tomé4, Reference Gaudichon, Bos and Morens44) and in pigs, with values of 93–97 %(Reference Deglaire, Bos and Tomé4, Reference de Vrese, Frik and Roos64).
Close agreement for digestibility between pigs and humans, in particular the high degree of correlation for true ileal amino acid digestibility values, encourages the use of linear regression equations to predict true ileal amino acid digestibility for humans based on pig data. Coefficients of such equations (Fig. 1) have been previously estimated based on controlled studies comparing pig and human digestibility, but these are mainly based on relatively highly digestible proteins(Reference Deglaire, Bos and Tomé4). Regression-based prediction equations need to be founded on a wider selection of protein sources, including those containing fibre and antinutritional factors.
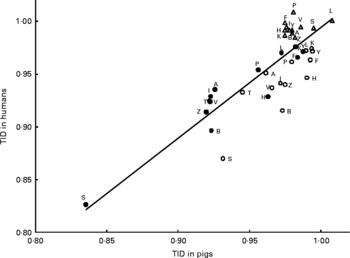
Fig. 1 Linear regression relationships between mean values for human and pig true ileal digestibilities (TID) of individual amino acids (y = 1·05x − 0·06; R2 = 0·68; Pslope < 0·001) for meals based on casein (○)(Reference Deglaire, Bos and Tomé4), hydrolysed casein (●)(Reference Deglaire, Bos and Tomé4) or on vegetable and animal proteins (△)(Reference Rowan, Moughan and Wilson39). A, alanine; B, asparagine or aspartic acid; F, phenylalanine; H, histidine; I, isoleucine; K, lysine; L, leucine; P, proline; S, serine; T, threonine; V, valine; Y, tyrosine; Z, glutamine or glutamic acid.
Although the pig is a fast-growing animal like the rat, its amino acid scoring pattern is much closer to that of the adult human than that of the growing rat (Table 4). As previously reviewed(Reference Guilloteau, Zabielski and Hammon62), digesta transit times and digestive efficiencies are comparable in pigs and humans. The digestive tract of the growing pig is anatomically and physiologically very similar to that of adult humans, as previously reported(Reference Guilloteau, Zabielski and Hammon62, Reference Miller and Ullrey66–Reference Pond and Houpt69). Gut fractional protein synthesis rates have been reported to be in the same range, with pig values of 43 to 51 % per day(Reference Simon, Munchmeyer and Bergner70–Reference Simon, Bergner and Munchmeyer71) and human values of 22 to 50 % per day(Reference Waterlow57). This suggests a similar mucosal protein renewal rate, which is supported by the better agreement between species for the amount of dietary nitrogen recycled within endogenous protein (as determined in the >10 kDa fraction of ultrafiltered digesta). The amount of dietary protein deemed to be recycled was only two times higher in pigs than in humans, with values of 21 % and 11 %, respectively(Reference Deglaire43), whereas in rats, the recycling was six times higher than that found in humans(Reference Deglaire, Moughan and Bos58).
Unlike the rat, the pig offers the possibility of collecting large samples of representative digesta(Reference Moughan and Rowan68), which is advantageous for chemical analyses, and allows continuous ileal digesta collection after surgical preparation, such as the insertion of a cannula or ileo-rectal anastomosis(Reference Hodgkinson and Moughan72). This enables study of the kinetics of postprandial absorption of dietary amino acids and nitrogen, which is of interest as post-absorptive metabolism has been reported to be similar in many aspects between pigs and humans(Reference Guilloteau, Zabielski and Hammon62). Ileal digesta can also be collected directly from the terminal ileum of animals post mortem, under conditions of terminal anaesthesia(Reference Fuller and Tomé1), such as in rats. This method is straightforward and may be seen as being more acceptable ethically than using cannulated pigs, as the need for surgery is avoided and there is no ongoing discomfort for the animal. However, this technique allows digesta collection (one sample only) at a single time post meal and thus there may be concerns about the representativeness of samples collected. In addition, for some diets insufficient amounts of digesta may be collected(Reference Fuller and Tomé1). Finally, the pig has the advantage of being a meal eater and readily consuming most foods eaten by humans. In addition, it has a similar dental anatomy to the human. Pig ileal amino-acid digestibility assays are, however, labour-intensive and relatively expensive compared with rat-based assays. Use of the minipig might remedy some of these logistical drawbacks as this special breed of pig consumes less food and needs less space.
Conclusion
Animal models are useful and necessary for studying dietary protein digestibility with application to humans, as human assays are technically, economically and ethically difficult to undertake. The growing pig and the growing rat have been widely used as animal models. Both species appear to afford good agreement with human protein digestibility values, especially for true ileal digestibility. However, the rat may be able to digest more poorly digestible proteins to a greater extent than humans and this needs to be investigated further. From a purely biological perspective, the pig is the preferred model due to greater anatomical, physiological and behavioural similarities. In terms of practical aspects, the use of the pig is more labour-intensive and more costly than the rat. Overall, it appears that the rat is an acceptable model for protein digestion in humans, especially for routine evaluation of true ileal protein digestibility, particularly as it is readily available, easily housed and cared for, and is a more economical option than the pig. Nevertheless, caution needs to be exercised when making estimations for poorly digestible proteins. Although the pig may be a more expensive and logistically-demanding model, it is, because of greater similarities in digestive physiology, a preferred model. Further, there is a greater body of literature where ileal protein digestibility has been specifically compared between humans and pigs, and close agreement has been found. It is recommended that a standardised true ileal digestibility assay be developed based on the growing pig and applied to a wide range of human foods and food ingredients to provide an international data base of true ileal amino acid and nitrogen digestibility. The growing rat may have particular application for the more rapid and less expensive screening of foods and for assessing the effects of processing and storage methods on ileal amino acid digestibility.
Acknowledgements
None of the authors had any conflict of interest. Both authors were involved in the writing of the manuscript.









