CVD is a leading cause of mortality worldwide. Atherosclerosis is a widely recognised underlying pathophysiological cause of CVD and is characterised by dyslipidaemia – an elevation in LDL-cholesterol and TAG and a reduction in HDL-cholesterol, either in isolation or in combination. Elevated total cholesterol and LDL-cholesterol are independent risk factors for CVD and interventions (both dietary and pharmaceutical) that aim to reduce cholesterol are central to health strategies to reduce CVD risk in populations.
Anthocyanins are natural plant pigments which confer the red, purple, blue and black colours of anthocyanin-rich foods, such as strawberries, blueberries, blackberries, aubergines and purple potatoes. Anthocyanins comprise an anthocyanidin flavonoid moiety that is O-linked to one or more sugar molecules. In Europe, anthocyanidin intake is reported to range from 3 to 32 mg/d based on European Food Safety Authority estimates of fruit and vegetable consumption( Reference Tennant, Davidson and Day 1 ) and it is estimated that about 90 % of the total anthocyanin intake is derived from soft fruits. Estimates of anthocyanin intakes in the US range from 4·6 mg/d (lower quintile) to 19·3 mg/d (upper quintile) based on reported fruit and vegetable consumption in large cohort studies( Reference Cassidy, O’Reilly and Kay 2 ). Although mean intakes of anthocyanins are quite low, single portions of anthocyanin-rich fruits and vegetables can provide very significant quantities of anthocyanins. For example, an 80 g portion of blackcurrants is reported to contain 574 mg anthocyanins( Reference Hollands, Brett and Radreau 3 ).
Epidemiological studies have shown that those consuming the highest quantities of anthocyanins are at a lower risk of developing CVD( Reference Jennings, Welch and Spector 4 , Reference Wang, Ouyang and Liu 5 ). These observations are substantiated by several human intervention trials providing evidence that ingestion of anthocyanin-rich foods and beverages( Reference Alvarez-Suarez, Giampieri and Tulipani 6 – Reference Erlund, Koli and Alfthan 9 ), and anthocyanin extracts( Reference Soltani, Hakimi and Asgary 10 – Reference Zhu, Ling and Guo 12 ) have beneficial effects on markers of CVD risk, with improvements in lipid profiles common across these studies. In a recent systematic review assessing the impact of purified anthocyanins and anthocyanin-rich extracts on biomarkers of CVD risk in both healthy and diseased individuals( Reference Wallace, Slavin and Frankenfeld 13 ), the authors conclude that supplementation with anthocyanins significantly improved LDL-cholesterol concentrations in hypercholesterolaemic individuals . However, anthocyanin supplementation did not consistently and significantly affect blood pressure, another biomarker of CVD risk.
Blood orange is a variety that is grown mostly in Mediterranean countries such as southern Italy and Spain. It is the anthocyanins, mainly cyanidin-3-O-glucoside, that is responsible for the red colouration of the flesh of the blood orange. Standard or ‘blonde’ oranges do not contain anthocyanins. The authors are only aware of a few studies that have investigated the effects of blood orange as a source of anthocyanins on biomarkers for CVD risk. To date, there have been few reports of the effects of blood orange on CVD biomarkers: two reports concerned with effects on platelet function and procoagulant activity( Reference Giordano, Coletta and Tamburrelli 14 , Reference Napoleone, Cutrone and Zurlo 15 ) and one on endothelial function, as assessed by flow mediated dilation of the brachial artery (FMD)( Reference Buscemi, Rosafio and Arcoleo 16 ). However, although blood orange consumption was shown to reduce platelet activity/coagulation( Reference Napoleone, Cutrone and Zurlo 15 ) and increase flow mediated dilatation( Reference Buscemi, Rosafio and Arcoleo 16 ), neither of these studies could demonstrate that the effects were due to the anthocyanins in blood orange. The aim of this study was to investigate for the first time the effects of daily ingestion of blood orange juice rich in anthocyanins on circulating LDL-cholesterol and other lipids/lipoproteins and on other biomarkers related to vascular function and CVD risk, using blonde orange juice without anthocyanins as a control. In addition, we quantified biomarkers of glycaemia before and after the participants had consumed orange juices for a total of 8 weeks.
Methods
Study population
A total of forty-five men and women aged 25–84 years with a waist measurement >94 cm (men) and >80 cm (women) were recruited in and around Norwich, UK. The study period was from August 2014 to June 2016. The exclusion criteria were as follows: smoking; medical conditions such as diabetes, heart disease, peripheral vascular disease, gastro-intestinal disease; prescribed and non-prescribed medication judged to affect the study outcome; some dietary supplements (e.g. fish oils); clinical results at eligibility assessment judged to affect the trial outcome or be indicative of a health problem. The trial was conducted in the Human Nutrition Unit at the Quadram Institute Bioscience, Norwich, UK (formerly Institute of Food Research). The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by both the Human Research Governance Committee of the Quadram Institute Bioscience and the East of England Cambridge and Hertfordshire Research Ethics Committee (reference no. 14/EE/0219). Each participant gave written informed consent before taking part in the trial. The trial is registered with clinicaltrials.gov (reference NCT02195934).
Study design
The study was a randomised, open label, two-arm cross-over trial investigating the effects of 28 d ingestion of orange juice anthocyanins on biomarkers for CVD. The two treatments were: (i) 500 ml blood orange juice providing 50 mg anthocyanins/d and (ii) 500 ml blonde orange juice without anthocyanins.
Orange juice (2×250 ml) was ingested daily for 28 d with a minimum 3-week washout period between each of the treatments. Participants were advised to drink the juice with a meal/food. If this was not possible then, in line with recommendations from the British Dental Association, they were advised to drink the juice through a straw. To aid compliance, participants were provided with a checklist. Each time a drink was consumed, they were instructed to record this on the checklist. Compliance to treatment was assessed from the checklist. For 2 weeks before the start of each treatment period and for the 28-d treatment period, participants completely excluded from the diet some food sources that contribute significantly to total anthocyanin intake (e.g. berry fruits) and limited other food sources known to have cardio-protective effects (e.g. oily fish, dark chocolate, green tea). Limited food sources were completely excluded from the diet for 24 h before assessment days. Participants were also instructed to completely avoid consuming oranges for the duration of their participation on the trial. A list of prohibited and limited foods was given to the participants to aid compliance.
The primary outcome measure for this trial was LDL-cholesterol. Secondary outcome measures were other lipids/lipoproteins (total- and HDL-cholesterol and TAG), biomarkers of vascular activity and endothelial function (nitric oxide (NO), carotid-femoral pulse wave velocity (cf_PWV); brachial-ankle pulse wave velocity (ba_PWV); aortic blood pressure), glycaemic control (fasting glucose, fructosamine) and inflammation (high-sensitivity C-reactive protein (CRP)). Primary and secondary outcome measures were assessed before and after the 28-d treatment period. As it was expected that 500 ml of orange juice per d for a combined total of 56 d was a substantial ‘sugar load’, glycated Hb (HBA1c) status was also compared between eligibility assessment and the end of study treatments.
The order in which the participants ingested the two treatments was determined by a computer-generated sequence of letters (randomisation.com). Participants meeting the eligibility criteria were allocated sequentially to a treatment sequence upon enrolment by the study manager.
Orange juice
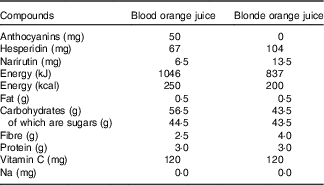
Both the standard and blood orange juice were commercially available not from concentrate products sold by Waitrose (‘100 % squeezed Sicilian blood orange juice’ and ‘Tangy and refreshing smooth orange juice’) and cartons/bottles were supplied directly by the manufacturer (AMC group). The composition of the juices is shown in Table 1. Juices were stored frozen (−20°C) and participants were instructed to defrost the juice in a refrigerator before consumption.
Table 1 Content of orange juice in a 500-ml serving
Biochemical markers for CVD
Whole blood was collected into EDTA, heparin and serum separating tubes (Becton Dickenson) and centrifuged accordingly. Serum lipids (total/HDL-/LDL-cholesterol and TAG), glucose, fructosamine and high-sensitivity CRP status were determined using an automated clinical chemistry analyser (Daytona plus; Randox). Plasma NO metabolites were quantified using a Sievers 280i NO analyser (Sievers Instruments Inc.). The 280i NOA reduces nitrite, nitrate and nitrosothiols to NO in a purge vessel which is then quantified according to the chemiluminescence signal released transiently within the instrument. Plasma samples were deproteinated with cold ethanol (1:2 dilutions) and centrifuged before analysis. Samples (20 µl) were injected onto the purge vessel and analysed in triplicate.
Blood pressure assessment
Measurements were conducted in a quiet room after a 10-min period of rest with the participant in a semi-supine position and the arm resting at heart level. Aortic systolic blood pressure (aSBP) and aortic diastolic blood pressure (aDBP) was measured using a Vicorder device (Smart Medical). Measurements were derived from a peripheral brachial blood pressure measurement and brachial pulse wave analysis using the vicorder in-built transfer function.
Arterial stiffness assessment
Cf_PWV and ba_PWV were measured with participants in the semi-supine position (30° angle) using a vicorder device. To determine cf_PWV an inflatable sensor (30 mm) was placed over the right carotid region and a BP cuff placed around the upper right thigh to measure the carotid and femoral pressure pulse waves, respectively. Path length was determined by measuring the distance (in cm) between the supra-sternal notch and the mid-point of the thigh cuff. The carotid sensor and thigh cuff were inflated (approximately 60 mmHg) and waveforms simultaneously recorded over 10–15 consecutive heartbeats. To determine ba_PWV, BP cuffs were placed around the ankle and the right upper arm. Path length was determined by measuring the distance between the supra-sternal notch and the mid-point of the ankle cuff. Both cuffs were inflated and pressure waveforms simultaneously recorded.
Statistical analysis
The primary outcome measure for this study was LDL-cholesterol. To detect a 0·162 mmol/l decrease in LDL-cholesterol at the 5 % significance level and with 80 % power, assuming sd 0·414 mmol/l in the paired post-intervention differences, forty-two participants were required to complete the study. Multilevel modelling( Reference Snijders and Bosker Roel 17 ) was used to analyse the response variables of interest. The following ‘explanatory variables’ were the same for all response variables: age, sex, BMI, order of diets given, diet (blonde orange juice and blood orange juice), time (pre- or post-), diet×time interaction and subject ID (as a random effect). Model diagnostics and best fit criteria were used to decide on the model structure and any necessary transformations and/or outlier removal. The R statistics package( 18 ) was used to analyse the data. Data were analysed on a per protocol basis. The difference in the concentrations of HbA1c at the end of the study were compared with the concentrations at eligibility assessment using the sign test. All data were considered significant if P<0·05. All data are presented as means and standard deviations.
Results
Of the forty-five participants randomised to treatment, forty-one completed the trial (twenty men and twenty-one women). One participant withdrew before commencing the trial because of illness; one participant self-withdrew part way through the first test period citing difficulties with study time commitments as the reason for withdrawal; one was withdrawn by the research team due to difficulties in obtaining blood samples; one developed chest pain and was hospitalised – this was not considered to be related to the trial, but the participant was withdrawn. Fig. 1 shows the flow of participants through the trial and Table 2 shows the baseline demographics and values for biomarkers of CVD at the start of the trial. Compliance to treatments was high with overall compliance of 99·5 and 99·4 %; blood orange and blonde orange juice treatments, respectively (juice doses taken as a proportion of intended total) and individual compliance of ≥92 %.
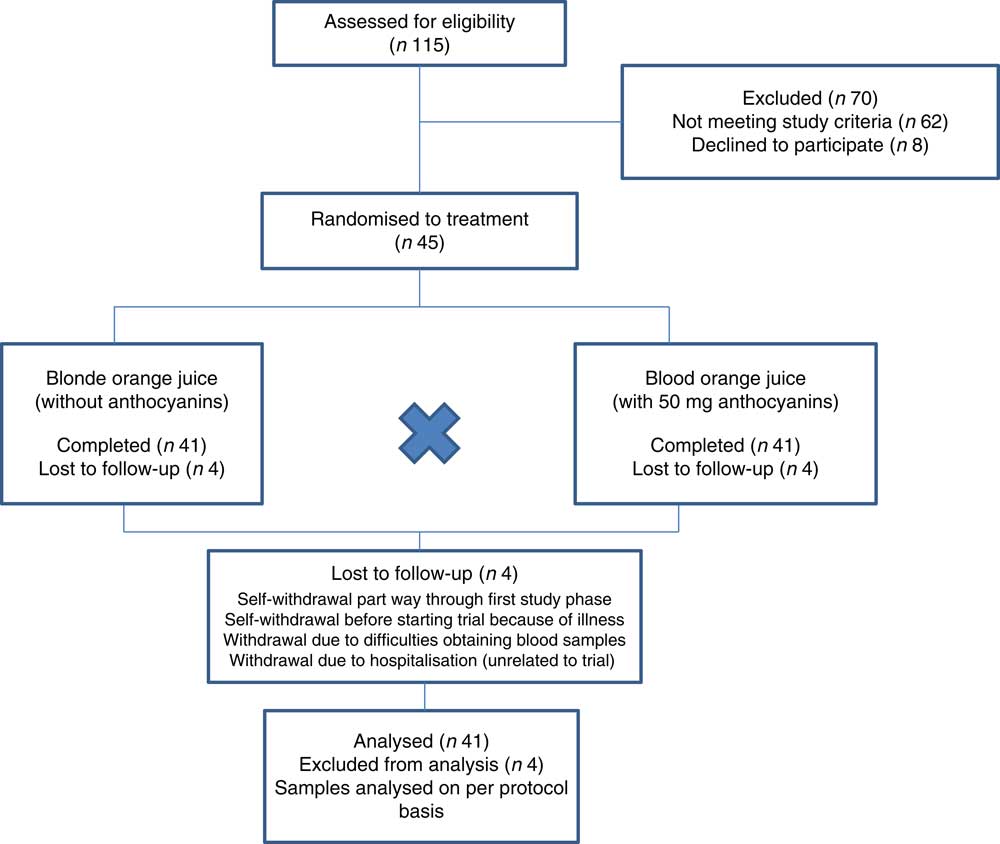
Figure 1 Flow of participants through trial.
Table 2 Baseline demographics and biomarkers of CVD at start of trial (Mean values and standard deviations; n 41)
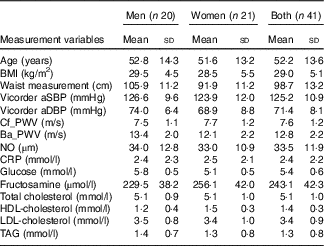
aSBP, aortic systolic blood pressure; aDBP, aortic diastolic blood pressure; Cf_PWV, carotid-femoral pulse wave velocity; Ba_PWV, brachial-ankle pulse wave velocity; NO, nitric oxide; CRP, C-reactive protein.
There were no significant differences at baseline between the treatment groups. After 28-d ingestion of blood orange juice and blonde orange juice, no significant differences between treatments were observed in aSBP, aDBP, circulating lipids (total-/HDL-/LDL-cholesterol and TAG), cf_PWV, ba_PWV, CRP, NO, glucose and fructosamine (Table 3). All values were within reported physiological ranges – the primary outcome measure (LDL-cholesterol) was also assessed with stratification by age (<42; 42–51; 52–61 and >61 years), but no significant difference between treatments was observed.
Table 3 Changes in CVD risk markers 28 d after ingestion of orange juice without anthocyanins (blonde orange juice) and with anthocyanins (blood orange juice) (Mean values and standard deviations)
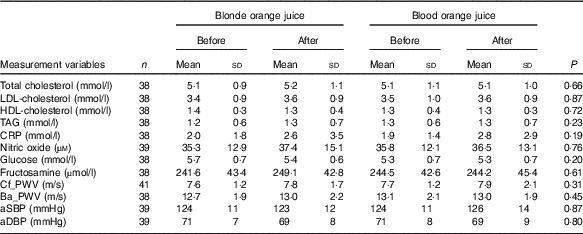
CRP, C-reactive protein; Cf_PWV, carotid-femoral pulse wave velocity; Ba_PWV, brachial-ankle pulse wave velocity; aSBP, aortic systolic blood pressure; aDBP, aortic diastolic blood pressure.
To assess the effects of juice ingestion on HbA1c status, post study concentrations of HbA1c were compared with the HbA1c concentrations determined at eligibility assessment. No significant difference in HbA1c between the two time points was observed (P=0·3915).
Discussion
We hypothesised that daily ingestion of 500 ml blood orange juice containing anthocyanins for 28 d would decrease LDL-cholesterol compared with a blonde orange juice that did not contain anthocyanins. However, we did not observe a beneficial effect of blood orange juice on either LDL-cholesterol or any of the other biomarkers of vascular function and CVD risk that were measured.
In a recent systematic review conducted by Wallace et al.( Reference Wallace, Slavin and Frankenfeld 13 ) twelve randomised controlled trials (RCT’s) were assessed for the impact of purified anthocyanins and anthocyanin-rich extracts on biomarkers of CVD risk in both healthy and diseased individuals. Nine of the twelve trials included LDL-cholesterol as an outcome evaluated for statistical significance in the intervention group compared with the control group. Of these nine trials, five were conducted in healthy individuals or diseased individuals with optimal or near optimal serum lipids and four were conducted in targeted hyperlidaemic individuals. The mean serum total cholesterol and LDL-cholesterol across the five studies of normolipidaemic individuals before intervention was 5·2 and 3·2 mmol/l, respectively. Across the four studies of targeted hyperlidaemic individuals, the mean total cholesterol and LDL-cholesterol at baseline was 6·3 and 3·8 mmol/l, respectively. The authors concluded that anthocyanins significantly improved LDL-cholesterol among diseased individuals or those with elevated biomarkers but did not significantly affect other markers of CVD risk. Participants in our trial were recruited with a waist circumference measurement that was indicative of central obesity, which in turn is associated with intra-abdominal fat including visceral adipose tissue (VAT), as there is known to be a positive correlation between VAT and CVD risk factors( Reference Pouliot, Despres and Lemieux 19 ). By association we anticipated that our cohort would present with elevated serum lipid concentrations. The mean total- and LDL-cholesterol concentration for the cohort at the start of our trial was 5·1 (sd 1·0) and 3·4 (sd 0·9) mmol/l, respectively, levels in keeping with those values reported in the five trials( Reference Curtis, Kroon and Hollands 20 – Reference Hassellund, Flaa and Kjeldsen 24 ) included as part of the systematic review( Reference Wallace, Slavin and Frankenfeld 13 ) in which no significant reductions in LDL-cholesterol were observed. This may account for the lack of a significant effect of the blood orange juice anthocyanins on LDL-cholesterol in the study reported here.
The differences observed between studies in lipid modulation after anthocyanin ingestion could be ascribed to a variety of factors including length of trial, study population, type of anthocyanin and the daily dose ingested. Two major differences existed between our trial and those reporting significant reductions in LDL-cholesterol that were reported as part of the systematic review of Wallace et al.( Reference Wallace, Slavin and Frankenfeld 13 ). First, the daily dose of anthocyanins ingested was higher in three of the four trials( Reference Soltani, Hakimi and Asgary 10 – Reference Zhu, Ling and Guo 12 ) ranging from 90 to 320 mg anthocyanins/d compared with 50 mg/d in our own trial. Despite the importance of establishing saturation effects and absorption thresholds after anthocyanin ingestion, few trials report the dose–response effect, which makes it difficult to ascertain the quantity of anthocyanins required to elicit a biological effect such as reductions in circulating cholesterol. After feeding 25 and 50 g freeze-dried strawberry (FDS)/d providing 78 and 155 mg anthocyanins/d, for a 12-week period to obese individuals with marginally higher than optimal serum lipid concentrations( Reference Basu, Betts and Nguyen 7 ) (total and LDL-cholesterol 5·3 and 3·2 mmol/l, respectively), the authors report a significantly greater decrease in LDL-cholesterol after ingestion of the high dose FDS compared with the low dose FDS. However, when compared with the control groups the differences only remained significant at the highest dose of FDS tested. Conversely, in a different trial in which healthy individuals were fed 400 and 1200 mg dried cranberry juice/d, providing 2·6 and 7·8 mg anthocyanins/d, respectively, for a period of 4 and 8 weeks, no significant effect on LDL-cholesterol was observed at either of the two doses or time points tested( Reference Valentova, Stejskal and Bednar 25 ).
In Europe, the mean intake of anthocyanins has been estimated to range between 3 and 32 mg/d( Reference Tennant, Davidson and Day 1 ), indicating that the dose of anthocyanins ingested by participants in our trial was greater than the average quantities consumed as part of habitual diets. The anthocyanins in blood orange juice are bioavailable in humans as described by Giordano et al.( Reference Giordano, Coletta and Tamburrelli 14 ). One difference between our trial and those highlighted in the systematic review( Reference Wallace, Slavin and Frankenfeld 13 ) as causing a reduction in LDL-cholesterol, is the type of anthocyanins ingested. Anthocyanins are present in plants as glycosides and are structurally based on two benzoyl rings, A & B, coupled with a third heterocyclic C ring. Anthocyanins are classified according to the number and position of hydroxyl groups at the B ring, the degree of methylation of the hydroxyl groups and by the number of conjugated sugars attached to the molecule; one or more molecules of glucose, rhamnose, galactose or arabinose to give mono-, di-, or trisaccharide forms. The four trials in the systematic review( Reference Wallace, Slavin and Frankenfeld 13 ) that reported significant reductions in LDL-cholesterol, were in response to ingestion of anthocyanin extracts of bilberry and blackcurrant( Reference Qin, Xia and Ma 11 , Reference Zhu, Ling and Guo 12 ), and whortleberry( Reference Soltani, Hakimi and Asgary 10 , Reference Kianbakht, Abasi and Hashem Dabaghian 26 ) which primarily contain mono- and di-glycosylated delphinidin and malvidin anthocyanins. In contrast, participants in our trial were consuming almost exclusively the cyanidin-3-O-monoglucoside anthocyanin present in blood orange. Moreover, four of the five trials in the systematic review( Reference Wallace, Slavin and Frankenfeld 13 ) that did not cause a significant reduction in LDL-cholesterol were either in response to ingestion of primarily cyanidin type anthocyanins( Reference Curtis, Kroon and Hollands 20 , Reference Naruszewicz, Laniewska and Millo 23 ) or delphinidin type anthocyanins at doses comparable to those provided in the study reported here( Reference Gurrola-Diaz, Garcia-Lopez and Sanchez-Enriquez 21 , Reference Hansen, Marckmann and Dragsted 22 ). Anthocyanin absorption is affected by the nature of the sugar moiety which in turn could give rise to differences in its ability to exert a biological effect. A recent animal trial( Reference Overall, Bonney and Wilson 27 ) has investigated the metabolic effects of berries containing structurally different anthocyanins in a C57BL/6 mouse model of polygenic obesity. Here, the authors report that animal diets supplemented with blackberry and black raspberry (mono-glycosylated cyanidins), blackcurrant, (mono and diglycosylated cyanidins and delphinidins), maqui berry (diglycosylated delphinidins), concord grape (mono-glycosylated delphinidins and petunidins) and blueberry (mono-glycosylated delphinidins, malvidins and petunidins) showed a discrepancy in the biological activities between delphinidin/malvidin type anthocyanins v. cyanidin type anthocyanins that could be explained by differences in their structure and metabolism in the gut. This strengthens the notion that differences in anthocyanin type could, to some extent at least, explain the discrepancies observed between human intervention trials investigating the effects of anthocyanins on cholesterol.
Anthocyanins have been shown to significantly reduce central blood pressure and cf_PWV, a direct measure of arterial stiffness, in a cross sectional study examining the associations between flavonoid sub-class and biomarkers of CVD risk( Reference Jennings, Welch and Fairweather-Tait 28 ). This is in direct contrast to the results of our trial. However, when a food based analysis of the anthocyanin sub-class (e.g. wine and berries) was undertaken, only the reductions in cf_PWV remained significant( Reference Jennings, Welch and Fairweather-Tait 28 ). There are limited published data from trials reporting the effects of anthocyanin-rich foods and beverages on measures of arterial stiffness, and those that do show a significant effect on cf_PWV tend to be from RCT conducted in individuals previously diagnosed with a cardiovascular condition( Reference Dohadwala, Holbrook and Hamburg 29 ).
Although this study was not primarily designed to investigate the possible effects of orange juice consumption on biomarkers of glycaemia, it does provide some useful data. The participants on the study reported here consumed 500 ml of 100 % orange juice for a total of 8 weeks, which provided on average 44 g of sugars and 941 kJ/d (225 kcal/d) (Table 1). There is a continuing debate about the role of free sugars in causing adverse health effects including obesity, dysregulated metabolic states such as diabetes, CVD and cancer. WHO define free sugars as ‘monosaccharides and disaccharides added to foods and beverages by the manufacturer, cook or consumer, and sugars naturally present in honey, syrups, fruit juices and fruit juice concentrates’. The World Health Organization( 30 ) has called on countries to reduce the free sugars intake of adults and children to <10 % of total daily energy intake although this was based on data from observational studies of the incidence of dental caries. Dietary recommendations in the USA (Dietary Guidelines Advisory Committee( 31 )), the UK (Scientific Advisory Committee on Nutrition( 32 )) and Canada (Heart and Stroke Foundation Canada( 33 )) recommend to reduce energy content from added sugars (=free sugars) to <10, <5 and <10 %, respectively. These recommendations were not solely based on prevention of dental caries, but also on reducing energy intakes and evidence of adverse health effects of consuming excess sugars. On the other hand, a systematic review and meta-analysis of prospective cohort studies investigating fruit juice intake and risk of type 2 diabetes showed that higher intakes of sweetened fruit juice was significantly associated with risk of developing type 2 diabetes (relative risk=1·28, P=0·02), but no association was observed for higher intakes of 100 % (unsweetened) fruit juice (relative risk=1·03, P=0·62). In the study reported here, we saw no significant effect of consuming a large dose of sugar-rich 100 % orange juice daily for 8 weeks on circulating HbA1c, and neither did we see any significant increases in fasting glucose or fructosamine after 4 weeks consumption of juice compared with baseline.
One of the main strengths of this study was the cross-over design approach. Participants acting as their own control minimised the influence of confounding variables when assessing the effects of the treatments. In addition, the daily dose of anthocyanins ingested by participants in this study is a dose that is easily achievable as part of a normal diet. This is important from a public health perspective. A potential limitation of this study is that it was conducted in predominantly overweight Caucasian adults and the same results may not be relevant to other population groups such as non-Caucasians and adults of normal weight. In addition, whilst our study population were at higher risk of dyslipidaemia (as determined by abdominal circumference) the mean plasma total cholesterol concentration of the study group was only minimally hyperlidaemic (5·1 mmol/l). Thus, our data do not preclude the possibility that effects of blood v. standard orange juice might occur in participants with more elevated total cholesterol (e.g. >6·0 mmol/l).
In conclusion, our data show that daily ingestion for 28 d of 50 mg anthocyanins derived from blood orange juice does not reduce LDL-cholesterol or any of the other biomarkers of vascular function and CVD risk measured, and nor did it alter biomarkers of glycaemia. Whether anthocyanin dose or type is important in terms of bioactivity in humans, is yet to be elucidated.
Acknowledgements
The authors thank Dr Jack Dainty for assistance with statistical analysis of the data, and the AMC group (30100 Espinardo-Murcia, Spain) for kindly providing the Sicilian blood orange and standard orange juices that were used in this study.
This research has received funding from the European commission (FP7-ATHENA; grant no. 245121)) and the Biotechnology and Biological Sciences Research Council (UK) through an Institute Strategic Programme Grant (‘Food and Health’; grant no: BB/J004545/1) to the Quadram Institute Bioscience (formerly Institute of Food Research). The European Commission and the Biotechnology and Biological Sciences Research Council had no role in the design, analysis or writing of this article.
Author contributions were as follows: P. A. K. designed the research and had primary responsibility for final content; W. J. H., J. F. D., C. N. A., M. S. W. and N. P.-M. conducted the research; W. J. H. and P. A. K. wrote the manuscript. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.








