Introduction
Mercury is a powerful neurotoxin that poses significant health risks to humans and wildlife (D'Itri and D'Itri Reference D'Itri and D'Itri1978, Wolfe et al. Reference Wolfe, Schwarzbach and Sulaiman1998). Anthropogenic inputs of mercury into air, water, and soil have resulted in a two- to threefold increase in global deposition over the past 200 years (Driscoll et al. Reference Driscoll, Han, Chen, Evers, Fallon Lambert, Holsen, Kamman and Munson2007), with heavily populated urban and industrial areas often exhibiting the most elevated environmental mercury levels (Landis et al. Reference Landis, Vette and Keeler2002, Dennis et al. Reference Dennis, Clair, Driscoll, Kammen, Chalmers, Shanley, Norton and Kahl2005). However, mercury's persistence in the atmosphere and ability to travel great distances from points of origin has allowed it to become a truly global pollutant, even contaminating areas without local sources (e.g. Antarctica; Wilson et al. Reference Wilson, Steenhuisen, Pacyna and Pacyna2006).
Primary sources of anthropogenic mercury emissions include coal and oil combustion, lead, zinc, steel, and cement production, gold mining, and waste incineration and disposal (Driscoll et al. Reference Driscoll, Han, Chen, Evers, Fallon Lambert, Holsen, Kamman and Munson2007, Pacyna et al. Reference Pacyna, Pacyna, Steenhuisen and Wilson2006). Total mercury emissions, and the contribution of each source, differ greatly among countries and continents and between the northern and southern hemispheres. Over the past decade, multiple international conventions have been formed to identify and implement strategies for effective reductions in mercury emissions. Most of this activity has occurred in developed countries in Europe and North America. As a result, recent emissions trends show a general reduction in these areas, whereas emissions continue to rise in most developing countries (Pacyna et al. Reference Pacyna, Pacyna, Steenhuisen and Wilson2006).
At the regional scale, local patterns of mercury deposition across the landscape can vary widely. Areas where humans, fish, and wildlife are at increased risk of mercury exposure are not necessarily areas of high mercury deposition (Evers et al. Reference Evers, Han, Driscoll, Kamman, Goodale, Lambert, Holsen, Chen, Clair and Butler2007). Several landscape characteristics influence the degree of mercury accumulation, conversion to methylmercury, and exposure to biota in an area (Driscoll et al. Reference Driscoll, Han, Chen, Evers, Fallon Lambert, Holsen, Kamman and Munson2007). This spatial heterogeneity at small scales complicates identification of areas where mercury may be particularly harmful; hence, widespread monitoring networks are needed. When hotspots and the most direct sources of contamination are successfully identified, large reductions in local emissions can have quick and significant effects at this scale (Davis et al. Reference Davis, McClenahen and Hutnik2007, Driscoll et al. Reference Driscoll, Han, Chen, Evers, Fallon Lambert, Holsen, Kamman and Munson2007, Evers et al. Reference Evers, Han, Driscoll, Kamman, Goodale, Lambert, Holsen, Chen, Clair and Butler2007).
Birds are considered excellent bio-indicators of environmental mercury contamination (Evers et al. Reference Evers, Burgess, Champoux, Hoskins, Major, Goodale, Taylor, Poppenga and Daigle2005) and sometimes serve in part as the basis for drafting mercury regulation policies or provide measures of success following policy implementation (e.g. NYSDEC 2006, Driscoll et al. Reference Driscoll, Han, Chen, Evers, Fallon Lambert, Holsen, Kamman and Munson2007). As such, mercury levels in birds have been widely recorded around the world (Gochfeld Reference Gochfeld1980, Burger et al. Reference Burger, Parsons, Benson, Shukla, Rothstein and Gochfeld1992, Reference Burger, Laska and Gochfeld1993, Reference Burger, Kennamer, Lehr Brisbin and Gochfeld1997; Burger and Gochfeld Reference Burger and Gochfeld1991, Reference Burger and Gochfeld1993; Janssens et al. Reference Janssens, Dauwe, Bervoets and Eens2001). However, relatively few studies have investigated the actual consequences of observed mercury levels on free-living birds’ condition, fitness, or survival. Fewer still have examined species outside North America and Europe. Beyond their limited geographical diversity, studies of mercury in birds have been disproportionately focused on particular taxa, foraging guilds and trophic levels, ecosystems, and life-history stages. Consequently, our understanding of the threats of mercury to birds remains incomplete. Here, I briefly summarize the documented effects of mercury on birds, identify information gaps, and subsequently suggest priority topics for future investigation. Readers are directed elsewhere for comprehensive reviews of the known effects of mercury on birds (Eisler Reference Eisler1987, Thompson Reference Thompson, Beyer, Heinz and Redmond-Norwood1996, Wolfe et al. Reference Wolfe, Schwarzbach and Sulaiman1998, Scheuhammer et al. Reference Scheuhammer, Meyer, Sandheinrich and Murray2007), as the focus of this commentary is on the relationships between mercury and avian biology that remain poorly understood.
Adverse effects of mercury in birds
Mercury is most available and harmful to birds and other biota in the form of methylmercury (Thompson and Furness Reference Thompson and Furness1989, Driscoll et al. Reference Driscoll, Han, Chen, Evers, Fallon Lambert, Holsen, Kamman and Munson2007). Inorganic mercury is most readily converted to methylmercury under anaerobic conditions in marine or freshwater systems such as wetlands, lakes, and reservoirs, although recent evidence suggests significant methylation may also occur in terrestrial systems (Rimmer et al. Reference Rimmer, McFarland, Evers, Miller, Aubry, Busby and Taylor2005, Driscoll et al. Reference Driscoll, Han, Chen, Evers, Fallon Lambert, Holsen, Kamman and Munson2007). Methylmercury bioaccumulates up food chains, reaching the most toxic levels in animals at upper trophic positions. It follows that predatory species associated with aquatic habitats are at the greatest risk of methylmercury accumulation (Evers et al. Reference Evers, Burgess, Champoux, Hoskins, Major, Goodale, Taylor, Poppenga and Daigle2005).
Most investigations into the effects of mercury on birds have examined aspects of reproduction. Earlier studies commonly compared some measure of reproductive success across populations of birds with different average mercury concentrations and implied a causal relationship without necessarily accounting for potential confounding factors (e.g. Fimreite Reference Fimreite1974). Some studies have compared mercury levels observed in wild birds to levels shown to cause adverse effects in laboratory animals (e.g. Burger and Gochfeld Reference Burger and Gochfeld1997). Perhaps the most thorough studies of mercury on bird reproduction are those that investigate mercury levels and multiple reproduction parameters within individual animals (e.g. Evers et al. Reference Evers, Taylor, Major, Taylor, Poppenga and Scheuhammer2003, Reference Evers, Savoy, DeSorbo, Yates, Hanson, Taylor, Siegel, Cooley, Bank, Major, Munney, Mower, Vogel, Schoch, Pokras, Goodale and Fair2008). Mercury's effects on birds are far-ranging and its impacts on reproduction are usually the end-point of more direct effects on behaviour, neurology, and physiology.
Behaviour
Field and laboratory studies have found high mercury burdens in birds to result in lethargy, loss of appetite, and reduced motivation to forage. Bouten et al. (Reference Bouten, Frederick, Spalding and McGill1999) noted that dosing captive Great Egrets Ardea alba with methylmercury altered activity budgets, with dosed birds spending more time sitting and less time engaged in activities such as preening than control birds. Dosed birds additionally had reduced appetites and showed less motivation to hunt fish. Spalding et al. (Reference Spalding, Frederick, McGill, Bouton and McDowell2000a) and Sepulveda et al. (Reference Sepulveda, Williams, Frederick and Spalding1999) found a similar relationship between mercury and appetite in captive and wild Great Egrets, respectively. In Common Loons Gavia immer, Evers et al. (Reference Evers, Savoy, DeSorbo, Yates, Hanson, Taylor, Siegel, Cooley, Bank, Major, Munney, Mower, Vogel, Schoch, Pokras, Goodale and Fair2008) documented a significant inverse relationship between methylmercury burden and time spent engaged in high-energy activities such as foraging for chicks and themselves, locomotion, and agonistic interactions with others. They also documented aberrant incubation behaviour by parents. Insufficient time spent incubating and foraging for chicks were suspected to be among the significant causes of the reduced reproductive success observed in loons with relatively high mercury levels. Nocera and Taylor (Reference Nocera and Taylor1998) found loon chicks with the highest mercury levels to ride on the backs of their parents least often, and therefore expend more energy and expose themselves to predation more than is typical.
Neurology
Mercury is a neurotoxin that affects coordination in humans and many other animals (D'Itri and D'Itri Reference D'Itri and D'Itri1978, Wolfe et al. Reference Wolfe, Schwarzbach and Sulaiman1998). Ataxia is a common behavioural characteristic of birds suffering from relatively high mercury burdens (Finley et al. Reference Finley, Stickel and Christensen1979, Laties and Evans Reference Laties and Evans1980, Bouten et al. Reference Bouten, Frederick, Spalding and McGill1999). Spalding et al. (Reference Spalding, Frederick, McGill, Bouton, Richey, Schumacher, Blackmore and Harrison2000b) found captive Great Egrets dosed with methylmercury to suffer severe lesions to nervous system tissues, resulting in slower reaction times to various stimuli and difficulty flying, perching, and standing. Heinz and Hoffman (Reference Heinz and Hoffman1998) similarly found captive Mallards Anas platyrhynchos dosed with methylmercury to have difficulty standing, although dosing levels exceeded those typically observed in nature.
Laties and Evans (Reference Laties and Evans1980) showed methylmercury to affect the operant discrimination ability of birds, when the success rates of Wood Pigeons Columbia livia trained to complete a task significantly declined after methylmercury dosing.
Physiology
Mercury has been associated with reductions in egg production, egg size, hatching success, and fertility in several field and laboratory studies (Fimreite Reference Fimreite1974, Finley and Stendell Reference Finley and Stendell1978, Heinz and Hoffman Reference Heinz and Hoffman1998, Evers et al. Reference Evers, Taylor, Major, Taylor, Poppenga and Scheuhammer2003; but see Thompson et al. Reference Thompson, Hamer and Furness1991). Each is expected to ultimately result in reduced reproduction rates within wild populations.
Methylmercury may reduce the production of haem, a component of haemoglobin that binds to and transports oxygen in blood (Olsen et al. Reference Olsen, Evers and DeSorbo2000). Consequently, birds with high methylmercury levels may have reduced oxygen carrying capacity and poor ability to sustain high-intensity exercise such as long-distance flight. Olsen et al. (Reference Olsen, Evers and DeSorbo2000) found Common Loons with relatively high methylmercury levels had shorter underwater dive durations during foraging and attributed this reduced ability to hold breath to low haem production.
Energy for feather growth is provided by protein in muscle tissue where much of ingested methylmercury is deposited. Additionally, methylmercury has a high affinity for keratin and thus a large proportion of ingested mercury travels to growing feathers (Fournier et al. Reference Fournier, Karasov, Kenow, Meyer and Hines2002). It is not surprising that methylmercury may therefore affect feather development or function in some capacity. Evers et al. (Reference Evers, Savoy, DeSorbo, Yates, Hanson, Taylor, Siegel, Cooley, Bank, Major, Munney, Mower, Vogel, Schoch, Pokras, Goodale and Fair2008) documented increased flight feather asymmetry in loons with higher methylmercury burdens and suggested this may significantly impact individual fitness by decreasing flight efficiency.
Reproduction
Many of the effects of mercury on birds’ behaviour, neurology, and physiology indirectly influence reproductive success. Reduced egg production, egg size, and hatching success will reduce fecundity in wild birds (Meyer et al. Reference Meyer, Evers, Hartigan and Rasmussen1998, Burgess and Meyer Reference Burgess and Meyer2008, Evers et al. Reference Evers, Savoy, DeSorbo, Yates, Hanson, Taylor, Siegel, Cooley, Bank, Major, Munney, Mower, Vogel, Schoch, Pokras, Goodale and Fair2008). Aberrant parenting behaviour, such as that observed in Common Loons (Evers et al. Reference Evers, Savoy, DeSorbo, Yates, Hanson, Taylor, Siegel, Cooley, Bank, Major, Munney, Mower, Vogel, Schoch, Pokras, Goodale and Fair2008), will likely lead to low chick survival. Aberrant behaviour of chicks may further contribute to reduced survival (Nocera and Taylor Reference Nocera and Taylor1998), although in most cases it appears methylmercury has little direct effect on chicks due to their ability to sequester it in newly grown feathers and away from living tissue (Fournier et al. Reference Fournier, Karasov, Kenow, Meyer and Hines2002, Merrill et al. Reference Merrill, Hartigan and Meyer2005, Longcore et al. Reference Longcore, Haines and Halteman2007).
Survival
Determining the effect of mercury on adult bird survival is complicated by contributions from countless other variables that can simultaneously influence survival rates of wild animals and confound analyses. Additionally, the large, long-term datasets required for powerful survival estimates are seldom available. It is likely that for these reasons, there have been few attempts to measure the effects of mercury on survival in wild birds.
Thompson et al. (Reference Thompson, Hamer and Furness1991) reported no effect of mercury concentration on the return of Great Skuas Catharacta skua to their breeding colony the following year. Meyer et al. (Reference Meyer, Evers, Hartigan and Rasmussen1998) similarly noted the likelihood of Common Loons returning to their breeding grounds between years was unaffected by their total mercury levels, suggesting mercury may not reduce adult survival. However, loons are long-lived birds and long-term effects of mercury exposure may not manifest in reduced survival until later in life. Mitro et al. (Reference Mitro, Evers, Meyer and Piper2008) used 10 years of Common Loon mark-recapture/re-sight data from North America to model survival rates and investigate a potential effect of mercury. The study found no significant effect on survival; although, despite the large dataset, statistical power was only sufficient for detecting differences in survival > 3% between high and low total mercury level groups. The authors nonetheless argue that even 3% reductions in survival could cause significant population declines in long-lived species such as loons.
Knowledge gaps and priorities for future research
The majority of mercury research done to date has been focused on select taxonomic groups, foraging guilds and trophic levels, ecosystems, countries and regions, and life history stages. As such, large knowledge gaps remain and further assessment of mercury's risks to birds is needed (Table 1).
Table 1. Relatively well- and under-studied areas concerning the effects of mercury on birds.
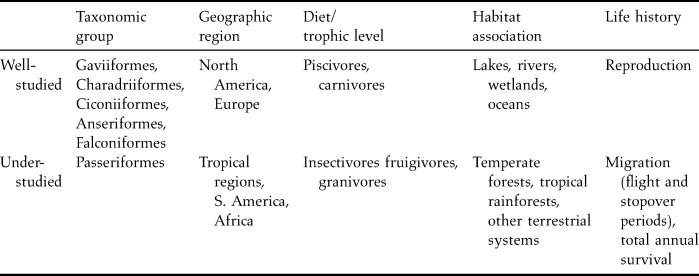
Taxonomy
Nearly all mercury research on birds (field and laboratory) has examined species that inhabit aquatic systems, as they are always expected to be at greatest risk for methylmercury exposure and accumulation. These primarily include species of loons (Gaviiformes), wading birds (Ciconiiformes), seabirds (Charadriiformes), and waterfowl (Anseriformes). Although these groups are widely considered to be the most vulnerable to environmental mercury, they are not necessarily the only groups of birds being impacted. Full risk assessments will require consideration of birds beyond those traditionally associated with mercury contamination (Burger et al. Reference Burger, Kennamer, Lehr Brisbin and Gochfeld1997).
Songbirds (Passeriformes) comprise the largest Order of birds, with approximately 5,400 species representing over half of all described bird species (Gill Reference Gill1995). Many of these species are experiencing steep population declines and are of conservation concern (Robins et al. Reference Robins, Sauer, Greenberg and Droege1989, Askins et al. Reference Askins, Lynch and Greenburg1990, Sanderson et al. Reference Sanderson, Donald, Pain, Burfield and van Bommel2006, Butcher and Niven Reference Butcher and Niven2007). Yet, until recently the threat of mercury to songbirds has been largely overlooked because of their low trophic positions and usual association with terrestrial habitats (Burger et al. Reference Burger, Kennamer, Lehr Brisbin and Gochfeld1997, Reference Burger, Bowman, Wolfenden and Gochfeld2004).
Reports of detectable mercury and methylmercury concentrations in wild songbirds are increasing (Janssens et al. Reference Janssens, Dauwe, Bervoets and Eens2001, Adair et al. Reference Adair, Reynolds, McMurry and Cobb2003, Evers et al. Reference Evers, Burgess, Champoux, Hoskins, Major, Goodale, Taylor, Poppenga and Daigle2005, Rimmer et al. Reference Rimmer, McFarland, Evers, Miller, Aubry, Busby and Taylor2005, Shriver et al. Reference Shriver, Evers, Hodgman, MacCulloch and Taylor2006, Brasso and Cristol Reference Brasso and Cristol2008, Cristol et al. Reference Cristol, Brasso, Condon, Fovargue, Friedman, Hallinger, Monroe and White2008), but it is uncertain whether the levels observed are great enough to be detrimental to songbird health or fitness. Brasso and Cristol (Reference Brasso and Cristol2008) found female Tree Swallows Tachycineta bicolor with high total mercury burdens produced fewer fledglings than individuals with lower total mercury levels. Similarly, Longcore et al. (Reference Longcore, Haines and Halteman2007) found total mercury concentrations were higher in some unhatched than hatched Tree Swallow eggs. In contrast, several studies have found total mercury levels in free-living songbirds to be lower than levels shown to cause adverse effects in published laboratory or field studies of non-passerine species (Custer et al. Reference Custer, Custer, Dickerson, Allen, Melancon and Schmidt2001, Reference Custer, Custer, Goatcher, Melancon, Matson and Bickham2006; Adair et al. Reference Adair, Reynolds, McMurry and Cobb2003, Hothem et al. Reference Hothem, Trejo, Bauer and Crayon2008), suggesting the songbirds examined were not accumulating harmful levels of mercury. However, the validity of this approach is questionable because threshold effect levels may in fact be lower in passerines than other Orders that are commonly used by such studies for reference (Longcore et al. Reference Longcore, Haines and Halteman2007, Tsipoura et al. Reference Tsipoura, Burger, Feltes, Yacabucci, Mizrahi, Jeitner and Gochfeld2008, Heinz et al. Reference Heinz, Hoffman, Klimstra, Stebbins, Kondrad and Erwin2009). For example, Tsipoura et al. (Reference Tsipoura, Burger, Feltes, Yacabucci, Mizrahi, Jeitner and Gochfeld2008) noted total mercury levels of unhatched Marsh Wren Cistothorus palustris eggs were significantly greater than those of successfully hatched eggs even though the unhatched eggs’ mercury levels were below those reported to be toxic to various non-passerine species.
Despite the growing interest in mercury accumulation in passerines, it remains poorly understood what concentrations are necessary to cause the sub-lethal negative effects that have been well-documented in other Orders (lethal toxicity levels in some passerines are reported by Finley et al. Reference Finley, Stickel and Christensen1979). Captive dosing studies of passerines similar to those conducted on aquatic birds (e.g. Spalding et al. Reference Spalding, Frederick, McGill, Bouton and McDowell2000a,Reference Spalding, Frederick, McGill, Bouton, Richey, Schumacher, Blackmore and Harrisonb) are needed to establish lowest observable adverse effect levels and would improve interpretation of past and future measurements of mercury in free-living songbirds. Additional field studies that examine mercury and multiple health or fitness parameters within individual songbirds (e.g. Brasso and Cristol Reference Brasso and Cristol2008) should also be a priority (Rimmer et al. Reference Rimmer, McFarland, Evers, Miller, Aubry, Busby and Taylor2005).
Foraging guild and trophic level
Mercury bioaccumulates up food chains as methylmercury and reaches the highest concentrations in predatory species. For this reason mercury has been commonly studied in piscivores, and to a lesser-extent, carnivores such as hawks, falcons, and owls (Thompson Reference Thompson, Beyer, Heinz and Redmond-Norwood1996), while other guilds have been comparatively ignored. Although species at lower trophic positions are expected to have lower levels of total mercury and methylmercury, these levels may still be great enough to cause adverse effects. This may be particularly true for insectivores that are not as high on food chains as piscivores or carnivores, but may nevertheless occupy trophic positions where methylmercury can sufficiently bioaccumulate. This is evident in some of the studies of insectivorous passerines referenced above. For example, Evers et al. (Reference Evers, Burgess, Champoux, Hoskins, Major, Goodale, Taylor, Poppenga and Daigle2005) found blood mercury levels of insectivorous Red-winged Blackbirds Agelaius phoeniceus exceeded those of various piscivorous bird species. Yet, additional research remains needed to conclusively determine if the total mercury or methylmercury levels observed in insectivorous birds are great enough to cause adverse effects. Captive dosing studies would help elucidate the consequences of the mercury and methylmercury levels that have been documented in wild insectivores. Further mercury monitoring in wild insectivores is also needed, as at present, very few species and geographic locations have been examined. Primarily frugivorous and granivorous species do not appear to be at risk of significant mercury/methylmercury accumulation (Burger et al. Reference Burger, Kennamer, Lehr Brisbin and Gochfeld1997, Rimmer et al. Reference Rimmer, McFarland, Evers, Miller, Aubry, Busby and Taylor2005, Fredricks et al. Reference Fredricks, Fedynich, Benn and Ford2009), but studies of such species are few.
Ecosystems
Aquatic systems are most efficient at converting inorganic mercury into methylmercury, thereby placing aquatic species at increased risk of methylmercury exposure (Evers and Clair Reference Evers and Clair2005, Evers et al. Reference Evers, Burgess, Champoux, Hoskins, Major, Goodale, Taylor, Poppenga and Daigle2005, Reference Evers, Han, Driscoll, Kamman, Goodale, Lambert, Holsen, Chen, Clair and Butler2007, Driscoll et al. Reference Driscoll, Han, Chen, Evers, Fallon Lambert, Holsen, Kamman and Munson2007). Among aquatic systems, methylmercury availability is believed to increase from marine to riverine to lake and wetland systems (Evers et al. Reference Evers, Burgess, Champoux, Hoskins, Major, Goodale, Taylor, Poppenga and Daigle2005). Total mercury and methylmercury concentrations in bird species that inhabit each of these systems have been widely documented (Muirhead and Furness Reference Muirhead and Furness1988, Thompson et al. Reference Thompson, Furness and Walsh1992, Burger and Gochfeld Reference Burger and Gochfeld1993, Thompson Reference Thompson, Beyer, Heinz and Redmond-Norwood1996, Evers et al. Reference Evers, Burgess, Champoux, Hoskins, Major, Goodale, Taylor, Poppenga and Daigle2005, Reference Evers, Han, Driscoll, Kamman, Goodale, Lambert, Holsen, Chen, Clair and Butler2007, Reference Evers, Savoy, DeSorbo, Yates, Hanson, Taylor, Siegel, Cooley, Bank, Major, Munney, Mower, Vogel, Schoch, Pokras, Goodale and Fair2008). In contrast, terrestrial habitats and their wildlife have received little attention concerning mercury (Lacher and Goldstein Reference Lacher and Goldstein1997, Rimmer et al. Reference Rimmer, McFarland, Evers, Miller, Aubry, Busby and Taylor2005, Driscoll et al. Reference Driscoll, Han, Chen, Evers, Fallon Lambert, Holsen, Kamman and Munson2007).
The mechanisms for methylation of inorganic mercury in terrestrial systems, such as temperate forests, are poorly understood. It is unclear whether the majority of methylmercury present in forest foliage is directly deposited from the atmosphere, or originates as dry-deposition inorganic mercury and is then methylated within leaves (Rimmer et al. Reference Rimmer, McFarland, Evers, Miller, Aubry, Busby and Taylor2005, Driscoll et al. Reference Driscoll, Han, Chen, Evers, Fallon Lambert, Holsen, Kamman and Munson2007). Few studies have examined the accumulation of methylmercury by strictly terrestrial birds whose diet is not closely linked to aquatic habitats. Rimmer et al. (Reference Rimmer, McFarland, Evers, Miller, Aubry, Busby and Taylor2005) found detectable methylmercury levels in five species of insectivorous passerines nesting in high elevation montane forests of northeastern USA and southeastern Canada. In a Virginia, USA watershed, Cristol et al. (Reference Cristol, Brasso, Condon, Fovargue, Friedman, Hallinger, Monroe and White2008) found forest birds with diets of terrestrial origin had significantly greater total mercury concentrations than aquatic-feeding birds; however, the study sites were in a historically contaminated industrial area. These studies demonstrate that temperate forests can potentially expose birds to significant amounts of mercury and methylmercury, but additional support would be beneficial.
Tropical forests are well-known for their high biodiversity and conservation priority. Thorough studies of mercury in these systems are lacking (Lacher and Goldstein Reference Lacher and Goldstein1997). Methylation processes are unclear, but some characteristics of tropical forests suggest methylation efficiency and methylmercury exposure to wildlife may be great. The long, wide, and complex food webs that are typical of tropical systems may provide great biomagnification potential, and the drastic variations in soil moisture are expected to enhance methylation processes (Burger Reference Burger1996).
Burger et al. (Reference Burger, Laska and Gochfeld1993) did not find detectable concentrations of mercury among various passerine species in the rainforests of Papua New Guinea. In contrast, Rimmer et al. (Reference Rimmer, McFarland, Evers, Miller, Aubry, Busby and Taylor2005) found total mercury burdens of Bicknell's Thrushes Catharus bicknelli during winter on tropical Hispaniola to be significantly greater than during their breeding season in the temperate northeastern USA and southeastern Canada - the regions of greatest mercury deposition in North America.
As in temperate areas, mercury contamination and biotic uptake in tropical systems likely varies dramatically at regional and local scales. Widespread documentation and monitoring of mercury accumulation in tropical areas is sorely needed (Burger Reference Burger1996, Lacher and Goldstein Reference Lacher and Goldstein1997). Lacher and Goldstein (Reference Lacher and Goldstein1997) declared mercury research in the tropics among the highest priorities in ecotoxicology. Sources of anthropogenic mercury emissions in temperate and tropical countries differ greatly, with fossil fuel combustion often accounting for most emissions in the former and gold mining accounting for most emissions in the latter (Pfeiffer et al. Reference Pfeiffer, Lacerda, Salomons and Malm1993, Lacher and Goldstein Reference Lacher and Goldstein1997, Porcella et al. Reference Porcella, Ramel and Jernelov1997, Pacyna et al. Reference Pacyna, Pacyna, Steenhuisen and Wilson2006). This limits the application of our knowledge of mercury transport and fate, and strategies for effective emissions controls in temperate systems to that in tropical systems.
Geography
Mercury appears to have been studied in birds in North America and Europe more than any other continents. Within North America, the northeastern USA and southeastern Canada have been examined most extensively (see Evers and Clair Reference Evers and Clair2005, Evers et al. Reference Evers, Burgess, Champoux, Hoskins, Major, Goodale, Taylor, Poppenga and Daigle2005, Driscoll et al. Reference Driscoll, Han, Chen, Evers, Fallon Lambert, Holsen, Kamman and Munson2007). Elsewhere in the world, mercury levels in birds have been measured in a wide variety of areas (e.g. Gochfeld Reference Gochfeld1980, Burger et al. Reference Burger, Parsons, Benson, Shukla, Rothstein and Gochfeld1992, Reference Burger, Laska and Gochfeld1993; Burger and Gochfeld Reference Burger and Gochfeld1991, Reference Burger and Gochfeld1993), but reports are sporadic. Comprehensive mercury monitoring is lacking outside North America and Europe, especially in many developing countries where emission trends are rising (Burger Reference Burger1996, Lacher and Goldstein Reference Lacher and Goldstein1997, Pacyna et al. Reference Pacyna, Pacyna, Steenhuisen and Wilson2006). China alone accounts for 28% of annual global mercury emissions, mainly due to its reliance on coal to meet increasing energy demands (Pacyna et al. Reference Pacyna, Pacyna, Steenhuisen and Wilson2006). Impacts on Asian birds and other biota should be closely monitored.
Life history
Prior studies of mercury's effects on birds have been limited to general aspects of health, survival, and reproduction. Mercury's effects on other life history stages, such as migration, remain largely unknown and should be a priority for future research. Migration is the most challenging period in a migratory bird's life cycle and has been estimated to account for as much as 85% of total annual adult mortality (Sillett and Holmes Reference Sillett and Holmes2002). Further, migration performance may have carry-over effects into subsequent seasons (Newton Reference Newton2006). Events occurring during migration can thus directly and indirectly influence bird population levels.
Migration for many species consists of alternating bouts of long-distance flying and rapid refuelling during stopovers (Moore et al. Reference Moore, Gauthreaux, Kerlinger, Simons, Martin and Finch1995). The adverse neurological, physiological, and behavioural effects of mercury that have been documented in non-migrating birds provide ample evidence to suspect that high mercury burdens would hinder migrants during both of these activities. Flight feather asymmetry (Evers et al. Reference Evers, Savoy, DeSorbo, Yates, Hanson, Taylor, Siegel, Cooley, Bank, Major, Munney, Mower, Vogel, Schoch, Pokras, Goodale and Fair2008) and reduced oxidative carrying capacity of blood (Olsen et al. Reference Olsen, Evers and DeSorbo2000) caused by mercury could conceivably weaken flight efficiency, whereas lethargy, ataxia, and reduced appetite/motivation to forage (Nocera and Taylor Reference Nocera and Taylor1998, Bouten et al. Reference Bouten, Frederick, Spalding and McGill1999, Sepulveda et al. Reference Sepulveda, Williams, Frederick and Spalding1999, Spalding et al. Reference Spalding, Frederick, McGill, Bouton and McDowell2000a) may affect stopover refuelling ability. It is also plausible that en route migrants experience surges in circulating methylmercury levels as a result of protein catabolism during long-distance flights. Methylmercury bound in muscle and other lean tissues may be re-mobilized into the bloodstream when protein is broken down to provide flight energy, water, or citric acid cycle intermediaries (Jenni and Jenni-Eiermann Reference Jenni and Jenni-Eiermann1998), consequently delivering additional methylmercury to the brain and other nervous system components. How mercury affects bird migration remains unstudied and warrants greater attention.
The categories above are of course not mutually exclusive and broad overlap occurs. For example, terrestrial, insectivorous, migratory, passerines fall into most of these categories. The research needs identified are also not exhaustive. Not included above is the need for laboratory studies of birds that more closely mimic mercury concentrations observed in nature. Laboratory studies should also more frequently measure and report mercury levels in the same tissues that are typically sampled in field studies, such as blood and feathers. Laboratory studies often only report mercury levels in organs measured after study subjects die or are euthanased which complicates interpretation of wild bird mercury levels that are measured non-invasively (Burger and Gochfeld Reference Burger and Gochfeld1997). The interaction of mercury with other contaminants in birds also needs to be further examined. Selenium, for example, may ameliorate some of mercury's effects (Thompson Reference Thompson, Beyer, Heinz and Redmond-Norwood1996, Heinz and Hoffman Reference Heinz and Hoffman1998) while worsening others (Heinz and Hoffman Reference Heinz and Hoffman1998), but the relationship between the two is not entirely clear (see also Cuvin-Aralar and Furness Reference Cuvin-Aralar and Furness1991, Khan and Wang Reference Khan and Wang2009).
Conclusion
Dramatic increases in world-wide mercury emissions have occurred over the past two centuries as a product of human population growth and industry. Environmental mercury contamination threatens human and wildlife populations. Reducing emissions is therefore a global concern, yet trends continue to rise in many parts of the world.
Birds are clearly vulnerable to mercury contamination. Much is known about mercury's effects on birds, but many relationships between mercury and bird biology remain poorly understood. The taxonomic groups, geographic ranges, life history stages, habitat associations, and foraging guilds of birds that are significantly threatened by mercury pollution need to be better identified. Successful bird conservation strategies are dependent on a comprehensive understanding of the threats facing populations. Additional research into how mercury is impacting birds will likely benefit conservation efforts.
Acknowledgements
This manuscript was improved by comments on earlier versions by H. A. L. Henry and S. B. Elbin. I am grateful to D. C. Evers for sparking my interest in the effects of mercury on birds. Support during preparation of the manuscript was provided by the University of Western Ontario and the Wildlife Conservation Society.





