Introduction
The East Asian-Australasian Flyway (EAAF) supports more waterbird species and is facing greater threats with more threatened species than any other flyway in the world (Kirby Reference Kirby2010, Wetlands International 2017). The shores of the Yellow Sea are a particularly important component of the EAAF, with internationally significant staging areas for at least 24 migratory shorebird species (Barter Reference Barter2002, Bamford et al. Reference Bamford, Watkins, Bancroft, Tischler and Wahl2008, MacKinnon et al. Reference MacKinnon, Verkuil and Murray2012). However, the tidal flat ecosystems along the Yellow Sea are disappearing at an alarming rate: 28% of tidal flats existing in the 1980s had disappeared by the late 2000s, indicating an annual rate of loss of 1.2% (MacKinnon et al. Reference MacKinnon, Verkuil and Murray2012, Ma et al. Reference Ma, Melville, Liu, Chen, Yang, Ren, Zhang, Piersma and Li2014, Murray et al. Reference Murray, Clemens, Phinn, Possingham and Fuller2014, Melville et al. Reference Melville, Chen and Ma2016). Meanwhile, measurable correlated population and survival declines of the shorebirds and other waterbirds that rely on intertidal areas as feeding habitat were detected (Amano et al. Reference Amano, Szekely, Koyama, Amano and Sutherland2010, Wilson et al. Reference Wilson, Kendall, Fuller, Milton and Possingham2011, Piersma et al. Reference Piersma, Lok, Chen, Hassell, Yang, Boyle, Slaymaker, Chan, Melville and Zhang2016). These studies highlight the question of whether these waterbirds can use alternative habitats, for example human-made saltpans (Hua et al. Reference Hua, Tan, Chen and Ma2015, Studds et al. Reference Studds, Kendall, Murray, Wilson, Rogers, Clemens, Gosbell, Hassell, Jessop, Melville, Milton, Minton, Possingham, Riegen, Straw, Woehler and Fuller2017).
Anthropogenic wetlands, created or extensively modified by humans, are rapidly expanding and replacing natural wetlands worldwide (Czech and Parsons Reference Czech and Parsons2002, Ma et al. Reference Ma, Cai, Li and Chen2010). Although anthropogenic wetlands cannot completely replace the functions of natural ones as waterbird habitat (e.g. Ma et al. Reference Ma, Li, Zhao, Jing, Tang and Chen2004, Desrochers et al. Reference Desrochers, Keagy and Cristol2008, Choi et al. Reference Choi, Gan, Hua, Wang and Ma2014, Sebastián-González and Green Reference Sebastián-González and Green2016), they can provide alternative or supplemental habitats for waterbirds. Examples of anthropogenic wetlands used by waterbirds include rice fields (Elphick Reference Elphick2000, Sanchez-Guzman et al. Reference Sanchez-Guzman, Moran, Masero, Corbacho, Costillo, Villegas and Santiago-Quesada2007), coastal grazing marshes (Milsom et al. Reference Milsom, Langton, Parkin, Peel, Bishop, Hart and Moore2000, Rhymer et al. Reference Rhymer, Robinson, Smart and Whittingham2010), extensive aquaculture ponds (Ma et al. Reference Ma, Jing, Tang and Chen2002, Li et al. Reference Li, Chen, Lloyd, Zhu, Shan and Zhang2013, Navedo and Fernández in press), and coastal saltpans (Warnock and Takekawa Reference Warnock and Takekawa1995, Masero et al. Reference Masero, Perez-Hurtado, Castro and Arroyo2000). Although the effective management of these anthropogenic wetlands can help to mitigate the adverse effects of natural wetland loss or degradation (reviewed by Ma et al. Reference Ma, Cai, Li and Chen2010, Navedo et al. Reference Navedo, Fernández, Valdivia, Drever and Masero2017), in the EAAF many salt ponds, for example, are being converted to modern aquaculture or industrial land (Flaherty and Karnjanakesorn Reference Flaherty and Karnjanakesorn1995, Sripanomyom et al. Reference Sripanomyom, Round, Savini, Trisurat and Gale2011).
In China, the total area of coastal saltpans increased from 1,639 km2 in 1985 to 2,413 km2 in 2010 and the total area of aquaculture ponds increased from 2,606 km2 to 12,099 km2 (Yao et al. Reference Yao, Ren, Wang, Wang and Deng2016). However, during this century the area of coastal saltpans decreased owing to competing land uses driven by urbanisation and economic growth (Li et al. Reference Li, Zhao, Zhou and Li2011).
Coastal saltpans are anthropogenic wetlands used for producing salt by the solar evaporation of seawater (Rocha et al. Reference Rocha, Fonseca, Masero and Ramos2016). They are often classified as ‘functional wetlands’ with a high biological richness and support large numbers of shorebirds and other waterbird groups worldwide (e.g. Masero Reference Masero2003, Sripanomyom et al. Reference Sripanomyom, Round, Savini, Trisurat and Gale2011, Houston et al. Reference Houston, Black, Elder, Black and Segal2012, Green et al. Reference Green, Sripanomyom, Giam and Wilcove2015, Rogers et al. Reference Rogers, Stamation, Loyn and Menkhorst2015). Their role in providing foraging and/or resting grounds for waterbirds varies according to species, time of year and tidal cycle. Many shorebirds feed on the tidal flats at low tide and move to adjacent supratidal saltpans to continue feeding or resting at high tide, while many others prefer to spend most of their time in the saltpans (Masero et al. Reference Masero, Perez-Hurtado, Castro and Arroyo2000, Dias Reference Dias2009). Likewise, waterbirds such as herons and gulls respond to the tide cycle in a similar way to shorebirds, and ducks and grebes use the supratidal ponds throughout the tide cycle (Warnock et al. Reference Warnock, Page, Ruhlen, Nur, Takekawa and Hanson2002). Coastal saltpans also provide breeding grounds for waterbirds (Ramírez et al. Reference Ramírez, Abdennadher, Sanpera, Jover, Wassenaar and Hobson2011, Reference Ramírez, Navarro, Afán, Hobson, Delgado and Forero2012, Rocha et al. Reference Rocha, Fonseca, Masero and Ramos2016).
The Nanpu Saltpan complex in Bohai Bay of the Yellow Sea, North China, is one of the largest coastal saltpans in the world, occupying an area of 290 km2 (Figure 1). In 2010, the adjacent intertidal zone was documented as a critical foraging ground for 62% of the Red Knot Calidris canutus, and 23% of the Curlew Sandpiper Calidris ferruginea flyway populations during northward migration (Rogers et al. Reference Rogers, Yang, Hassell, Boyle, Rogers, Chen, Zhang and Piersma2010, Yang et al. Reference Yang, Chen, Barter, Piersma, Zhou, Li and Zhang2011). In 2012, in the Nanpu Saltpans, large numbers of Curlew Sandpipers, Marsh Sandpipers Tringa stagnatilis, Black-tailed Godwits Limosa limosa, and White-winged Terns Chlidonias leucopterus were counted in the northward migration, comprising up to 11%, 1%, 3%, and 4% of their flyway populations, respectively (Hassell et al. Reference Hassell, Boyle, Slaymaker and Chan2012). However, these counts were only performed during the northward migration in the supratidal ponds, and they did not cover the whole of the Nanpu Saltpans. Therefore, we do not know the relative importance of these supratidal ponds in relation to the natural tidal flats, and it is likely that the true peak numbers present in these saltpans are higher than the peak numbers reported previously.
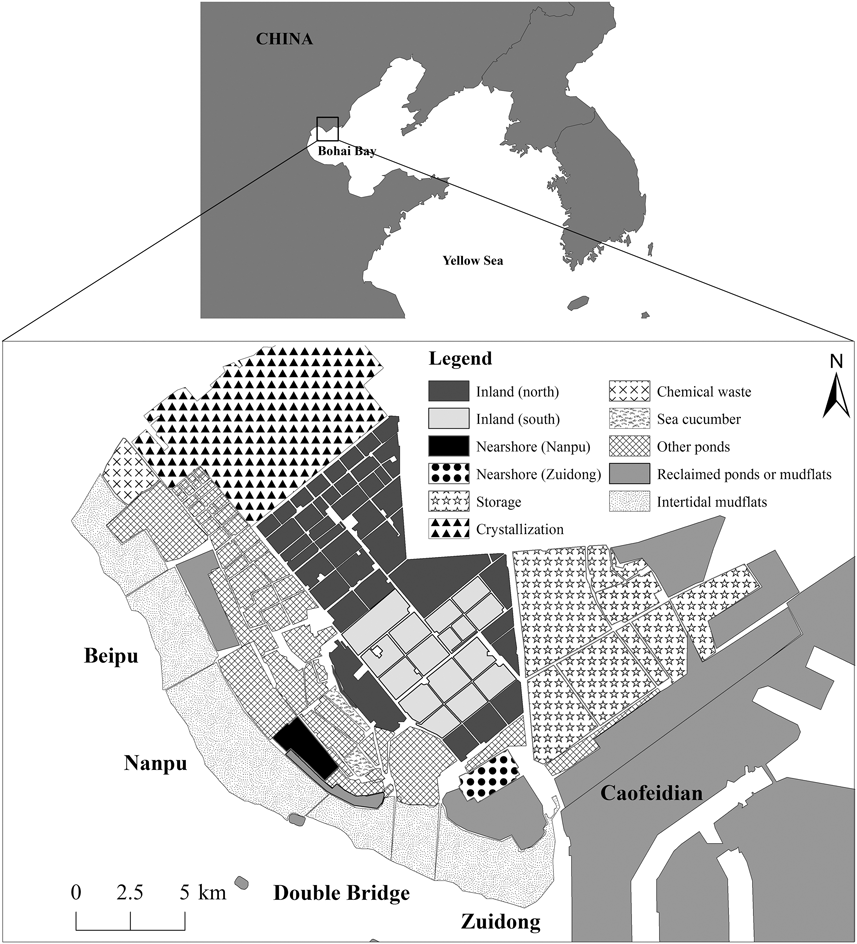
Figure 1. Map of Nanpu wetlands. The surveyed areas included inland and nearshore ponds and tidal flats. See legend and text for details.
Here, we investigate the role of the Nanpu Saltpans for waterbirds during the northward migration, southward migration and boreal winter. We report, for the first time, the waterbird numbers using the entire Nanpu Saltpans at both high and low tide, as well as on the adjacent tidal flats at low tide. These comparative counts will improve our understanding of how migratory waterbirds use the system and the current international importance of these unprotected saltpans for migratory waterbirds in the East Asian-Australasian Flyway.
Methods
Study area
The study area comprised most ponds available for waterbirds as feeding and/or roosting grounds in the Nanpu Saltpans (93 km2), as well as the adjacent tidal flats (57 km2) in the north of Bohai Bay (39°–12’N, 118°8’-28’E) and other supratidal anthropogenic habitats in the area such as shrimp and fish ponds (Figure 1). Similar to other industrial saltpans, the Nanpu Saltpans consist of shallow, interconnected pans of varying sizes separated by dikes. There are three types of ponds: storage, evaporation, and crystallization ponds (Figure 1). Briefly, seawater flows into the storage ponds with the rising tide and circulates through a series of evaporation ponds until it reaches many small crystallization ponds (Britton and Johnson Reference Britton and Johnson1987, Masero Reference Masero2003). The evaporation ponds are operated in two ways: one maintains a high water level (60–100 cm) and one maintains a low water level (40–60 cm) (salt worker Mr. Hu, pers. comm.). Shorebirds normally use water levels < 10 cm (Ntiamoa-Baidu et al. Reference Ntiamoa-Baidu, Piersma, Wiersma, Poot, Battley and Gordon1998). The low water level ponds can fall below 20 cm in the water transfer process, but the water in high water level ponds rarely falls to levels allowing waterbirds to feed. We focused our survey effort on the evaporation ponds because most waterbirds used these ponds during previous counts. We classified these ponds into two groups according to their distances to the tidal flats: inland ponds (86 km2; 2.4–18.0 km from the tidal flats) and nearshore ponds (Nanpu: 3.50 km2, Zuidong: 3.64 km2; 0.3–4.3 km from the tidal flats). Due to saltpan management, the south inland ponds had restricted access in northward migration but free access in southward migration, and the north inland ponds had free access throughout the year (Figure 1). The tidal flats are 1–3 km wide at low tide and are completely submerged from about two hours before high tide (Yang et al. Reference Yang, Chen, Barter, Piersma, Zhou, Li and Zhang2011). For the counts, the tidal flats were divided into four sectors: Beipu (14 km2), Nanpu (22 km2), Double Bridge (10 km2), and Zuidong (11 km2) (Figure 1).
Surveys
To survey the saltpans, we drove along the perimeter roads and counted from the roads or pond levees, while the tidal flats were surveyed from a dike close to the sea. Waterbirds were counted using telescopes (25–60 x magnification eyepieces) or binoculars (8 x 30). The schedule of waterbird counts and sample sizes are shown in Table 1. For more details, see Appendix S1 in the online supplementary materials.
Table 1. Waterbird numbers (mean ± SE) in Nanpu Saltpans and adjacent tidal flats. Range (minimum and maximum counts), date of peak counts, shorebird numbers in relation to maximum abundance (%), number of waterbird species using the study area, count area (km2) and number of counts per season, respectively, also are shown. See text for details.

* Inland ponds were surveyed independently of the tidal cycle and/or did not cover all ponds.
**Inland ponds were surveyed independently of the tidal cycle.
***Zuidong tidal flats were excluded from the counts.
Northward migration counts - Inland ponds were counted regularly at high and low tide (2014–2016); nearshore ponds were only counted at high tide (2015–2016) because few birds used them during low tide. Tidal flats were counted at low tide synchronously with the low tide counts in the inland ponds (2014–2016). Due to logistical constraints, on the tidal flats all waterbirds were counted in 2014, nine abundant or important shorebird species in 2015 and all shorebirds species in 2016. All counts in 2013 and a few counts in 2014 in inland ponds performed independently of the tidal cycle were also included (Table 1); i.e. a single count included the high-tide period but also part of the low-tide period. All inland pond counts during northward migration focused only on the northern inland ponds because the southern inland ponds had restricted access.
Southward migration and boreal winter counts - We counted the inland ponds and tidal flats in southward migration in 2013–2016 and in boreal winter of 2013. Due to logistical constraints, the tidal flats were counted before or after the counts on the inland ponds, and the counts in the saltpans were performed independently of the tidal cycle. Unfortunately, we did not count nearshore ponds during southward migration and boreal winter because we could not catch high tide in these ponds. The counts in the inland ponds also included the southern inland ponds in 2013 (southward migration and boreal winter) and in 2016 (southward migration).
During high tide, all tidal flats are submerged, so waterbirds have to move into saltpans for roosting or feeding (previous experience at the study site allowed us to know all large feeding and roosting sites). In each round of counts, the maximum number of a species in the study area was obtained from the high-tide counts in the saltpans or from the total number of the synchronised low-tide counts on tidal flats and in saltpans. To estimate a species’ habitat usage, the number in the saltpans or on the tidal flats was divided by the maximum number in the study area (times 100%). Bird names followed the BirdLife Checklist Version 9.1 (http://datazone.birdlife.org/species/taxonomy).
Foraging activity in the saltpans
The foraging activity of waterbirds in the saltpans during northward migration from 2014 to 2016 was calculated at both the low- and high-tide periods, following Masero et al. (Reference Masero, Perez-Hurtado, Castro and Arroyo2000) and Masero and Pérez-Hurtado (Reference Masero and Pérez-Hurtado2001). During the low- and high-tide counts we noted the activity of individuals as foraging or resting (including preening). For calculating the foraging activity in saltpans, the duration of the low-tide period in the saltpans was considered the same as the emersion period on the tidal flats during low tide; the rest, up to the completion of the tidal cycle, was considered to be high tide (tidal flats submerged period) (Masero and Pérez-Hurtado Reference Masero and Pérez-Hurtado2001). For a given species, the number of foraging individuals divided by the total number was its foraging percentage. The overall foraging percentage is based on the average of all species in each survey.
Data analyses
We described the abundance and richness of waterbirds in the Nanpu Saltpans and on the adjacent tidal flats. The abundance of waterbirds was expressed as the mean abundance (MA), maximum abundance (MaxA) and density (birds/km2). Waterbird richness was expressed as the number of species. To compare the relative use of the saltpans and tidal flats during low tide we used the densities of waterbirds. Many ponds were not used for feeding as the water levels were too high, so for calculating density in the saltpans, 58 km2 of ponds were selected, usually with birds feeding or roosting, with approximately 62% of the total saltpans studied (93 km2). Note that not all of the 58 km2 of the ponds were available for waterbirds during each survey, so this will lead to a conservative estimate of the density in saltpans. The area of the tidal flats used for the density calculation was 57 km2.
Internationally important species are defined as those that are present in numbers exceeding 1% of their flyway populations according to the Convention on Wetlands (Ramsar Convention 1971, Yang et al. Reference Yang, Chen, Barter, Piersma, Zhou, Li and Zhang2011). Waterbird flyway populations followed either Hansen et al. (Reference Hansen, Fuller, Watkins, Rogers, Clemens, Newman, Woehler and Weller2016) or Waterbird Population Estimates Fifth Edition (WPE5) (Wetlands International 2017).
To describe the community composition during each season and habitat, we calculated the percentage contributed by each species. Waterbirds that were too distant to identify to species level were not included. The northward migration community composition of the tidal flats was based only on the data of 2014, because in 2015 and 2016, not all waterbird species were counted.
After checking for normality by Kolmogorov-Smirnov tests, we used independent t-tests to test the differences between the densities in saltpans and tidal flats during low tide, and differences between mean foraging percentage in inland ponds and nearshore ponds during high tide. We used paired sample t-tests to test the total abundance in the saltpans (inland ponds or whole saltpans). In addition, we used non-parametric Mann-Whitney U-tests to compare the density of each species between the saltpans and tidal flats during low tide and Wilcoxon signed-rank tests to compare the abundance of each species between high tide and low tide in the saltpans (inland ponds or whole saltpans). Statistical analyses were carried out in IBM SPSS Statistics 20.0 for Windows (Armonk, NY: IBM Corp). All tests were two-tailed, and significance levels were set at P < 0.05. Values given are means ± SE.
Results
Northward migration started in early March and ended in early June, with the abundance and richness of waterbird species reaching their peaks in mid-May. Some birds remained throughout June - presumably subadults or weak birds that were unable to migrate further north. Southward migration started in late June and lasted until late November, peaking in late August (Figure 2). After most birds had moved on in southward migration, some Dunlin Calidris alpina, Grey Plovers Pluvialis squatarola, and Eurasian Curlews Numenius arquata overwintered in the study area.
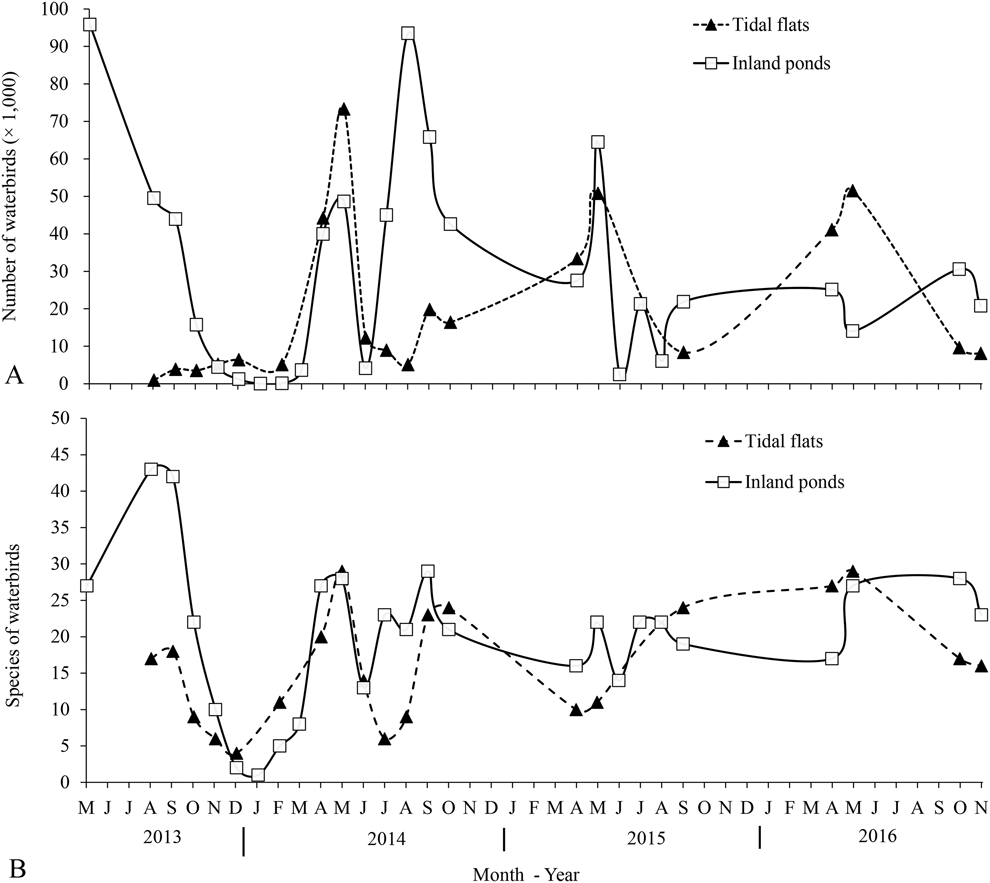
Figure 2. Bird numbers (A) and species (B) of waterbirds in inland ponds and tidal flats of Nanpu wetlands. Data are the maximum count and the maximum number of species found in each month.
A total of 89 waterbird species were recorded in the study area, among which 13 species were threatened or ‘Near Threatened’. Of these, 27 were recorded exclusively in saltpans and one exclusively in the adjacent tidal flats. In the saltpans, a total of 85 species occurred in inland ponds and 42 in nearshore ponds. During the northward migrations, the maximum number of waterbirds recorded in saltpans was 96,000 individuals on 16 May 2013 at high tide, and 64,490 on 19 May 2014 at low tide. The maximum number of waterbirds recorded in saltpans during the southward migration was 93,500 individuals on 23–24 August 2014 (Table 1). At low tide, the peak number of waterbirds on the tidal flats occurred on 13–14 May 2014 and 26–27 September 2014, with 73,000 and 20,000 individuals, respectively. Among the waterbirds, shorebirds were the most abundant group of birds both in the Nanpu Saltpans and on the tidal flats (Table 1).
A total of 27 species exceeded the 1% threshold of the flyway populations (Table 2). Among them, 23 species exceeded the threshold in the saltpans (13 species only in saltpans); 14 species exceeded the threshold on the tidal flats (four species only on tidal flats); and 10 species exceeded the threshold in both habitats. Twenty-three species exceeded the threshold in northward migration; 17 species exceeded the threshold in southward migration and three species did so in boreal winter. Of these species, 20 were shorebirds.
Table 2. Waterbirds exceeding 1% of the flyway estimate population (FEP) in Nanpu Saltpans and adjacent tidal flats. Maximum abundance (MaxA) were obtained from the high tide and low tide counts in Nanpu Saltpans, low tide counts on tidal flats, or the sum of both habitats during low tide synchronized counts (the percentage of FEP is shown between parenthesis after MaxA numbers). Peak counts during northward and southward migration in both saltpans and tidal flats also are shown.
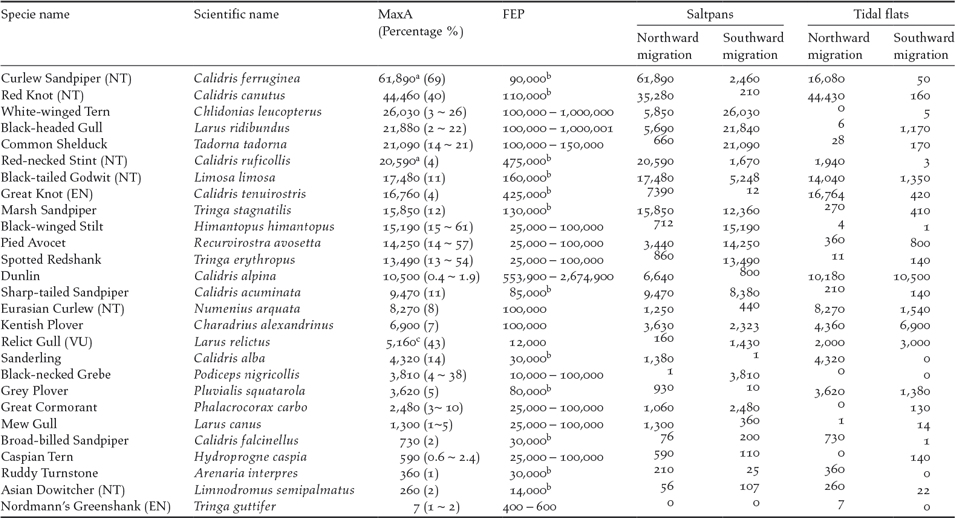
NT — Near Threatened, VU — Vulnerable, EN — Endangered, as listed by (IUCN 2015). a data from GFN; b FEP followed Hansen et al. (Reference Hansen, Fuller, Watkins, Rogers, Clemens, Newman, Woehler and Weller2016), other species followed WPE5. c MaxA counted during boreal winter on tidal flats.
The most abundant species in inland ponds were similar between high and low tides. The most abundant species in the inland ponds were different from the nearshore ponds, and the latter were similar to the tidal flats. The most abundant species were different between northward migration and southward migration, both in the saltpans and tidal flats (Appendix S2).
During northward migration, the density of waterbirds on the tidal flats was higher than in the saltpans during low tide (P = 0.001, t = -4.01, n = 12, df = 22). Species such as Red Knot and Great Knot Calidris tenuirostris had higher densities on the tidal flats than in the saltpans, whereas Black-tailed Godwit, Pied Avocet Recurvirostra avosetta, and Black-winged Stilt Himantopus himantopus had higher densities in the saltpans than on the tidal flats (Appendix S3).
The number of birds in the saltpans during high tide was higher than during low tide (P = 0.002, t = -5.18, n = 7, df = 6), but the number of birds on the inland ponds did not differ between high and low tide (P = 0.119, t = 1.68, n = 13, df = 12). Few birds used nearshore ponds during low tide (one count recorded 814 birds on 9 May 2016). When comparing the number in all the saltpans during high and low tide, Red Knot (P = 0.018, Z = -2.37), Great Knot (P = 0.018, Z = -2.37), Black-headed Gull Larus ridibundus (P = 0.028, Z = -2.20), Eurasian Curlew (P = 0.043, Z = - 2.02), and Bar-tailed Godwit Limosa lapponica (P = 0.028, Z = 2.20; all n = 7) were more abundant during high tide. For the inland ponds only, Black-tailed Godwit (P = 0.023, Z = -2.27), Common Shelduck Tadorna tadorna (P = 0.034, Z = -2.12), Dunlin (P = 0.028, Z = -2.20), Red Knot (P = 0.021, Z = -2.31), Red-necked Stint Calidris ruficollis (P = 0.019, Z = -2.35), and White-winged Tern (P = 0.028, Z = -2.20; all n = 13) were more abundant during high tide. Black-tailed Godwit, Curlew Sandpiper, Dunlin, Marsh Sandpiper, Pied Avocet, Red Knot, Red-necked Stint also were abundant during low tide (> 500 birds on average). During high tide, species like Red Knot and Great Knot were mainly present in nearshore ponds (Figure 3).
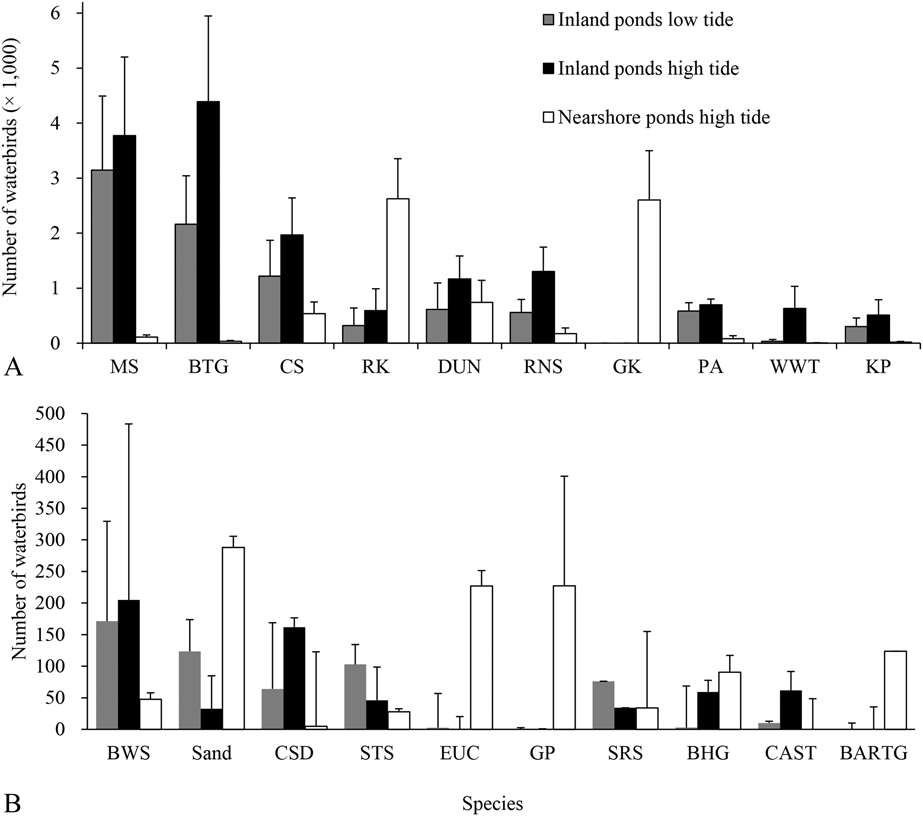
Figure 3. Bird numbers of the main species using inland and nearshore ponds during northward migration. High tide and low tide surveys were conducted in consecutive days or in days as close as possible. CAST= Caspian Tern, for other acronyms, see legend in Appendix S2. See text for methodological details.
During northward migration, most waterbirds in the inland ponds were actively feeding (except for Great Cormorant Phalacrocorax carbo which were just roosting), while the waterbirds (mainly shorebirds) in the nearshore ponds were roosting during high tide (Figure 4). However, Great Cormorants were found feeding in a storage pond in southward migration, which were dry in northward migration. The mean foraging percentage (average of each count) in the inland ponds was high during both high tide (80.7 ± 3.9%, n = 8) and low tide (91.2 ± 0.8%, n = 8). The foraging rate in the nearshore ponds is lower than in the inland ponds during high tide (10.4 ± 4.2%, n = 5, P < 0.001, t = -11.69, df = 11).
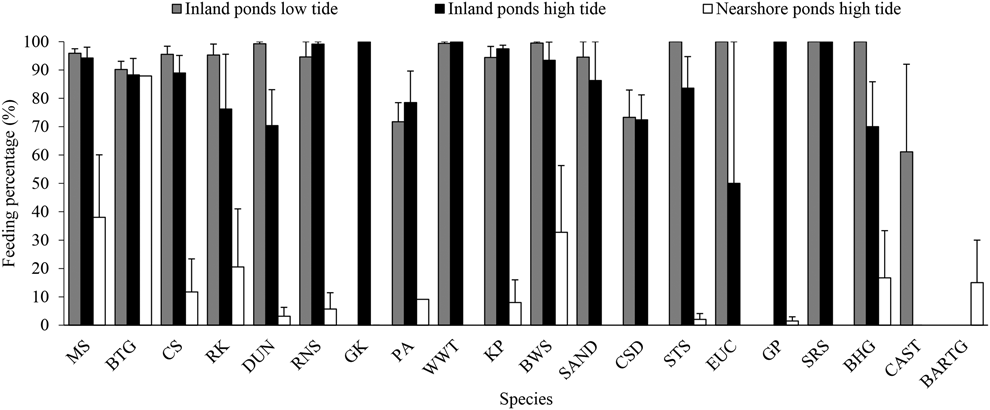
Figure 4. Feeding percentage of waterbirds in inland and nearshore ponds during northward migration. CAST = Caspian Tern, for other acronyms, see legend in Appendix S2. See text for methodological details.
The percentage of birds using different habitats and during different tides grouped into three categories (Figire 5). White-winged Tern, Pied Avocet, Marsh Sandpiper, Spotted Redshank Tringa erythropus, Black-winged Stilts, Common Shelduck, Black-tailed Godwit, Black-headed Gull, and Caspian Tern Hydroprogne caspia mainly used saltpans. Bar-tailed Godwit, Grey Plover, Eurasian Curlew, Great Knot, and Red Knot mainly used tidal flats, and they also used nearshore ponds during high tide. The remaining species occurred in both habitats (Figure 5).
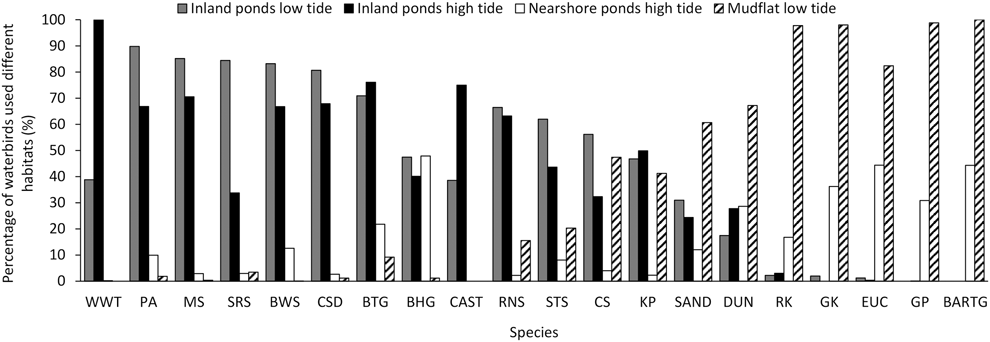
Figure 5. Percentage of birds using saltpans or tidal flats during northward migration at low or high tide. In these foraging behaviour data, there were no Great Knot and Grey Plover recorded during low tide, no Bar-tailed Godwit during both low tide and high tide in inland ponds, no White-winged Tern, Caspian Tern, and Spotted Redshank in nearshore ponds during high tide. The foraging percentages of Great Knot, Sanderling, Eurasian Curlew, and Common Shelduck in nearshore ponds were zero. CAST= Caspian Tern, for other acronyms, see legend in Appendix B. See text for methodological details.
Discussion
This study demonstrates the important role of the Nanpu Saltpans for migratory waterbirds using the East Asian-Australasian Flyway. Large numbers of many waterbird species used the Nanpu Saltpans as feeding and/or roosting sites during both the northward migration and southward migrations. A total of 89 species was recorded, of which 27 occurred in numbers of international importance, with 13 threatened or ‘Near Threatened’ species. Saltpans are not only supplementary feeding grounds for some waterbirds during high tide but also major feeding grounds for some species throughout the tidal cycle.
Habitat use
There are no natural wetlands in Nanpu, except the tidal flats, and all supratidal natural habitats had been converted into saltpans or aquaculture ponds. Both tidal flats and saltpans can provide feeding grounds for waterbirds; however, during high tide, when tidal flats are totally submerged, only saltpans can provide roosting and foraging grounds.
We showed that saltpans and tidal flats use patterns by waterbirds varied among species and seasonally. Thus, both habitats were used heavily by waterbirds during migration, but only a few species used Nanpu as a wintering area. The abundance of waterbirds recorded on southward migration 2015 and 2016 was lower than on southward migration 2013 and 2014, perhaps because there were fewer surveys in 2015 and 2016 than in 2013 and 2014. We did not count the nearshore ponds during southward migration and boreal winter, so the number of waterbirds in saltpans in both seasons may be higher than we recorded.
Migratory shorebirds were the most abundant group during northward migration. On southward migration, the percentage of shorebirds was lower than on northward migration, because the most abundant species on northward migration such as Red Knot, Great Knot and Curlew Sandpiper seldom used the study area on southward migration. We also noted during the counts that Pied Avocet, Black-winged Stilt, Kentish Plover, Common Redshank Tringa totanus, Little Tern Sterna albifrons, Common Tern Sterna hirundo, Gull-billed Tern Gelochelidon nilotica, and Eastern Spot-billed Duck Anas zonorhyncha breed in Nanpu Saltpans.
There are three hypotheses on why shorebirds feed in saltpans adjacent to natural intertidal areas: the ‘supplementary food hypothesis’, the ‘preference hypothesis’, and the ‘disturbance hypothesis’ (Masero et al. Reference Masero, Perez-Hurtado, Castro and Arroyo2000). Although the shellfish harvest on tidal flats may affect the foraging activities of waterbirds (Navedo and Masero Reference Navedo and Masero2007), the number of shellfish collectors was few (Yang et al. Reference Yang, Chen, Piersma, Zhang and Ding2016) and the tidal flats seem large enough for birds to feed free from humans (pers. obs.). Therefore, this hypothesis should be rejected.
The use of saltpans by Red Knots, Relict Gull Larus relictus, Dunlin, and Sanderling was consistent with the ‘supplementary food hypothesis’, as most birds used the tidal flats as foraging grounds and only fed in the saltpans at high tide. Other species such as Great Knot, Eurasian Curlew, Grey Plover and Bar-tailed Godwit almost only used the saltpans as roosting sites during high tide.
The ‘preference hypothesis’ seems to explain the foraging patterns of White-winged Tern, Pied Avocet, Marsh Sandpiper, Spotted Redshank, Black-winged Stilt, Common Shelduck, Black-tailed Godwit, and Black-headed Gull as they foraged in the inland ponds throughout the tidal cycle. The habitat use patterns of Curlew Sandpiper, Red-necked Stint, Sharp-tailed Sandpiper Calidris acuminata, and Kentish Plover were variable because many individuals fed on tidal flats during low tide, but many others also fed in saltpans during both high tide and low tide.
The findings of previous studies on the foraging use of coastal saltpans in other parts of the world were generally similar (e.g. Velasquez and Hockey Reference Velasquez and Hockey1992, Masero et al. Reference Masero, Perez-Hurtado, Castro and Arroyo2000, Warnock et al. Reference Warnock, Page, Ruhlen, Nur, Takekawa and Hanson2002, Dias Reference Dias2009). Black-tailed Godwit, Black-winged Stilt, Pied Avocet, Spotted Redshank, Kentish Plover (Masero et al. Reference Masero, Perez-Hurtado, Castro and Arroyo2000, Dias Reference Dias2009), Black-necked Stilt Himantopus mexicanus, and American Avocet Recurvirostra americana (Warnock et al. Reference Warnock, Page, Ruhlen, Nur, Takekawa and Hanson2002) preferred saltpans for foraging, while Grey Plover, Ruddy Turnstone Arenaria interpres, Bar-tailed Godwit, and Red Knot used saltpans as supplementary feeding grounds (Masero et al. Reference Masero, Perez-Hurtado, Castro and Arroyo2000, Dias Reference Dias2009).
The number and behaviour of waterbirds in inland ponds and nearshore ponds showed that these two groups of ponds had different functions for waterbirds in daytime. Inland ponds were mainly used as feeding grounds, whereas nearshore ponds were mainly used as high-tide roosting sites by shorebirds. High-tide roost sites are important to waterbirds because roosting sites can constrain their use of forage areas (Dias et al. Reference Dias, Granadeiro, Lecoq, Santos and Palmeirim2006, Rogers et al. Reference Rogers, Piersma and Hassell2006b). These constraints are particularly relevant in the context of Nanpu, since as stated previously, this area is densely populated and there are few sites available for roosting. We found a large number of shorebirds roosting in the nearshore ponds at high tide during daylight. These ponds are also used as high-tide roosting sites at night (Chris Hassell pers. comm.). Distance is an important factor affecting high-tide roosting site selection, and shorebirds using these ponds would be minimising the costs of flying from tidal flats to roost sites (Rogers et al. Reference Rogers, Battley, Piersma, Gils and Rogers2006a).
That saltpans may buffer the loss of natural habitats is supported by a large amount of evidence (Masero and Pérez-Hurtado Reference Masero and Pérez-Hurtado2001, Masero Reference Masero2003, Sripanomyom et al. Reference Sripanomyom, Round, Savini, Trisurat and Gale2011, Dias et al. Reference Dias, Lecoq, Moniz and Rabaga2014). The use patterns of saltpans and tidal flats by waterbirds found in this study indicate that the buffer role of saltpans varies among species and relies on the extent to which they use the saltpans. For the species preferring to feed in saltpans, such as Black-tailed Godwits and Black-winged Stilts, the loss of natural tidal flats could be largely compensated if the conditions in saltpans are suitable for feeding. For the species that use both saltpans and tidal flats, such as Curlew Sandpiper and Red-necked Stint, the loss of natural tidal flats can be partly compensated. This is important for those species that rely heavily on the Yellow Sea and whose flyway populations are dramatically declining (Studds et al. Reference Studds, Kendall, Murray, Wilson, Rogers, Clemens, Gosbell, Hassell, Jessop, Melville, Milton, Minton, Possingham, Riegen, Straw, Woehler and Fuller2017). However, for the species that seldom feed in the saltpans, such as Great Knot and Red Knot, the role of saltpans for feeding is limited, although they are important high-tide roosting sites.
International importance
Our findings confirmed the international importance of the Nanpu Saltpans for migrating waterbirds in the East Asian-Australasian Flyway. The Nanpu Saltpans provided feeding and roosting sites for large numbers of waterbirds and supported species of conservation concern. Thirteen threatened or ‘Near Threatened’ species on the IUCN Red List were recorded in saltpans or on tidal flats. One is Critically Endangered (Spoon-billed Sandpiper Calidris pygmaea). Four are ‘Endangered’ (Great Knot, Nordmann’s Greenshank Tringa guttifer, Far Eastern Curlew Numenius madagascariensis, and Oriental White Stork Ciconia boyciana). Two are ‘Vulnerable’ (Relict Gull and Saunders’ Gull Chroicocephalus saundersi). Six are ‘Near Threatened’ (Curlew Sandpiper, Red-necked Stint, Red Knot, Black-tailed Godwit, Eurasian Curlew, and Asian Dowitcher Limnodromus semipalmatus). The number of Great Knot, Nordmann’s Greenshank, Relict Gull, Curlew Sandpiper, Black-tailed Godwit, and Eurasian Curlew also exceeded 1% of their flyway populations (Hansen et al. Reference Hansen, Fuller, Watkins, Rogers, Clemens, Newman, Woehler and Weller2016, Wetlands International 2017). The Nanpu tidal flats and saltpans were listed in China’s top 21 priority coastal wetlands for waterbird conservation (Xia et al. Reference Xia, Yu, Millington, Liu, Jia, Wang, Hou and Jiang2017).
From 1993 to 2012, 10 shorebird taxa that refuel on the Yellow Sea tidal mudflats have declined by 65% (Studds et al. Reference Studds, Kendall, Murray, Wilson, Rogers, Clemens, Gosbell, Hassell, Jessop, Melville, Milton, Minton, Possingham, Riegen, Straw, Woehler and Fuller2017). These taxa include species that also showed a declining trend in this study, such as Curlew Sandpiper. The latter has been listed as a ‘Critically Endangered’ species in Australia under its Environment Protection and Biodiversity Conservation Act 1999 and as ‘Near Threatened’ on the IUCN Red List (IUCN 2017). This species has suffered an 80.5% population decline over the last three generations (a generation time of 7.6 years), with an annual rate of decline of 7.5% (Garnett et al. Reference Garnett, Szabo and Dutson2011, BirdLife International 2016a, Studds et al. Reference Studds, Kendall, Murray, Wilson, Rogers, Clemens, Gosbell, Hassell, Jessop, Melville, Milton, Minton, Possingham, Riegen, Straw, Woehler and Fuller2017). The maximum number of Curlew Sandpipers in the Nanpu Saltpans was 61,890 (May 16, 2013) recorded in a single pond (3.27 km2) (Hassell et al. Reference Hassell, Boyle, Slaymaker, Chan and Piersma2013), which comprised approximately 69% of the flyway population (Hansen et al. Reference Hansen, Fuller, Watkins, Rogers, Clemens, Newman, Woehler and Weller2016). Together with 20,580 Red-necked Stints and other species that fed in the same ponds, this single pond held more than 90,000 birds (Hassell et al. 2013 ). However, the maximum percentages of the estimated flyway population of Curlew Sandpiper recorded in the saltpans were only 26% (23,700 birds) in 2014, 39% (35,270) in 2015, and 18% (16,570) in 2016. Although four years of data are too short to detect population declines, given the high natural variability of shorebird numbers from year to year, the decline of the Curlew Sandpiper in this area is still of concern. This is because the environmental factors in the saltpans and tidal flats were similar for four years, and a decline was also found in other species, such as Red-necked Stint, Black-tailed Godwit, and Red Knot. Flyway populations of these species have declined sharply in recent decades (Studds et al. Reference Studds, Kendall, Murray, Wilson, Rogers, Clemens, Gosbell, Hassell, Jessop, Melville, Milton, Minton, Possingham, Riegen, Straw, Woehler and Fuller2017, Wetlands International 2017)
The maximum count of Black-tailed Godwit in the Nanpu Saltpans was 17,480 (22–23 April 2013), comprising approximately 11% of the flyway population (Wetlands International 2017). The global population of Black-tailed Godwits may have declined by approximately 25% since 1990 (BirdLife International 2016b). The maximum count of Black-tailed Godwits in northward migration declined during the study period, with 14,790 in 2015 and 10,180 in 2016.
Approximately 34,200 Red Knots (90% were the subspecies piersmai) were recorded feeding or roosting in a single pond (3.30 km2) on 29 May 2013, an estimated 51% of the world population of the subspecies piersmai (Hassell et al. Reference Hassell, Boyle, Slaymaker, Chan and Piersma2013). The summer survival of Red Knot has sharply declined since 2012, and coastal intertidal habitat loss in the Yellow Sea is the most likely cause (Piersma et al. Reference Piersma, Lok, Chen, Hassell, Yang, Boyle, Slaymaker, Chan, Melville and Zhang2016). The maximum number of Red Knot in the study area decreased from 44,430 (2014) to 30,750 (2015) and 15,340 (2016), suggesting that the summer survival of Red Knot is probably still declining. The flyway population of Red Knots in the study area sharply increased from 13% in 2007 to 62% in 2010 due to tidal flat loss in the surrounding area (Yang et al. Reference Yang, Chen, Barter, Piersma, Zhou, Li and Zhang2011), and the recent decrease suggests that their prospects are not good.
Conservation implications
Despite its conservation importance, the birds and their habitats in the Nanpu Saltpans have not been properly protected to date. Habitat loss and human disturbance are increasing in recent years in our study area. For instance, in the adjacent Tianjin Municipality, and Fengnan, Tangshan City, Hebei Province, more than 100 km2 of saltpans have been lost since Barter et al. (Reference Barter, Li and Xu2001) identified the international importance of this area for shorebirds (Melville et al. Reference Melville, Chen and Ma2016). Since 2010, at least 20 km2 in the Nanpu Saltpans have been converted to industrial land. In addition, another large project plans to use 100 km2 of the Nanpu Saltpans (Hebei Daily 2015). Moreover, construction of a new road across the Nanpu Saltpans starting in 2015 has cut through many ponds, including the one with 96,000 birds on 16 May 2013. This has caused sharp declines of waterbirds in these ponds (pers. obs.). Therefore, an urgent need exists to prevent further loss of the remaining saltpans and adjacent tidal flats. Other options include restoring lost habitats and modifying existing artificial coastal habitats to maximise shorebird foraging and roosting opportunities (Studds et al. Reference Studds, Kendall, Murray, Wilson, Rogers, Clemens, Gosbell, Hassell, Jessop, Melville, Milton, Minton, Possingham, Riegen, Straw, Woehler and Fuller2017). However, we do not suggest building more saltpans to replace natural feeding habitats because the saltpans attract different species to the tidal flats and this habitat is not suitable for all (Masero Reference Masero2003).
Today, some of the most important areas for shorebirds include coastal saltpans (Rogers et al. Reference Rogers, Stamation, Loyn and Menkhorst2015). It has been suggested that the presence of coastal saltpans could increase the carrying capacity of some estuarine systems for non-breeding shorebirds (Batty Reference Batty1992, Masero et al. Reference Masero, Perez-Hurtado, Castro and Arroyo2000). However, across the world, large saltpans are disappearing or are being abandoned due to changing economics driven by industrialisation, urbanisation and competing land uses (Masero Reference Masero2003). Saltpans do not only produce salt (see review by Rocha et al. Reference Rocha, Costa, Lucena-Filho, Bezerra, Medeiros, Azevedo-Silva, Araujo and Xavier-Filho2012). In the Nanpu Saltpans, storage and evaporation ponds are used for aquaculture, while in many other evaporation ponds the crustacean brine shrimp Artemia is harvested. All these activities can benefit local people. However, many ponds have high water levels to meet the needs of salt production (especially the high-water level evaporation ponds) or aquaculture, which limits their use by foraging shorebirds. Environmental conditions in the saltpans can affect the availability and accessibility of prey for waterbirds, so the available foraging area within the saltpans can vary several-fold (Dias Reference Dias2009). Therefore, saltpans need waterbird-friendly management to maintain their importance as feeding habitats for shorebirds (Rocha et al. Reference Rocha, Ramos, Paredes and Masero2017). For example, during the migration season, water levels and the available foraging area of the ponds should be manipulated whenever possible (Dias et al. Reference Dias, Lecoq, Moniz and Rabaga2014). For shorebirds, the best water level would be 0–10 cm, and for non-shorebirds 30–40 cm (Lei Reference Lei2017). Salinity should be maintained in several ranges, especially 20–60 ppt (for fish and fish-eating birds) and ∼140 ppt for shorebirds, waterfowl, gulls and terns (Warnock et al. Reference Warnock, Page, Ruhlen, Nur, Takekawa and Hanson2002).
This study clearly demonstrates the joint ecological function of The Nanpu Saltpan complex and adjacent tidal flats as a key staging area for waterbirds in the EAAF, and both urgently warrant protected status. Further studies in Nanpu complex should also focus on the potential impact of high-voltage power lines, since collision killed many birds in Nanpu Saltpans (pers. obs.), as well as on illegal eggs collection of breeding shorebirds such as Kentish Plover and Pied Avocet (Que et al. Reference Que, Chang, Eberhart-Phillips, Liu, Szekely and Zhang2015, Lei Reference Lei2017).
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270917000508
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Zhengwang Zhang, grant number 31572288), (Hong-yan Yang, grant number 31301885); and the Grant from WWF-China Programme. Theunis Piersma acknowledges funding from WWF-Netherlands and the Netherlands Organization for Scientific Research (NWO). We thank volunteers, Katarzyna Kucharska, Tong Mu, Jian Zhao and Peter Crighton’s great help. We thank the Global Flyway Network team (Chris Hassell, Adrian Boyle, Matthew Slaymaker, Bob Loos, supported by WWF-Netherlands and BirdLife Netherlands) who helped survey the tidal flats. Thanks to Jason Loghry, Jin Liu, Ginny Chen, Katherine Leung, Chao Yan, Fuxing Wu, Yu Liu, Pinjia Que, Yanjing Chang, Jiayu Liu, Ying Chen, David Melville, Jan Erik Nilsen, Terry Townshend, Per Alstrom, and friends from GSWJ birdwatching groups who helped with the surveys. Thanks also to Yajing Chang and Lei Guan who helped to produce the map. Professor Zhijun Ma reviewed an early version of the manuscript and gave valuable suggestions. Terry Townshend helped revise the language for the figures, table legends and appendices. We would also like to thank P. Atkinson and two anonymous referees for their comments on earlier versions of the manuscript.











