Introduction
The population in the European Union is predicted to increase to 523 million by 2060 with the proportion aged 65 years and over rising from 18.5 per cent in 2010 to 28.7 per cent in 2060 (Eurostat 2013). Thus, innovation in methods of managing functional decline and frailty are critical in balancing the needs of older adults with limited health-care resources (Rechel et al. Reference Rechel, Grundy, Robine, Cylus, Mackenbach, Knai and McKee2013). The European Innovation Partnership on Active and Healthy Ageing (EIP-AHA) was launched in 2011 to identify and remove barriers to innovation for active and healthy ageing. This paper explores the progress made in ageing and frailty research by partners within the EIP-AHA, with the aim of synthesising and sharing learning experiences to enable best practice in future frailty-based research and interventions.
Frailty
Frailty is a multi-dimensional clinical condition characterised by decreased physiological resilience and a weakened response to stressors (Rodríguez-Mañas et al. Reference Rodríguez-Mañas, Féart, Mann, Viña, Chatterji, Chodzko-Zajko, Gonzalez-Colaço Harmand, Bergman, Carcaillon, Nicholson, Scuteri, Sinclair, Pelaez, Van Der Cammen, Beland, Bickenbach, Delamarche, Ferrucci, Fried, Gutiérrez-Robledo, Rockwood, Rodríguez Artalejo, Serviddio and Vega2013). Consequently, frail individuals are predisposed to adverse events such as hospitalisation, institutionalisation and mortality (Clegg et al. Reference Clegg, Young, Iliffe, Rikkert and Rockwood2013).
Frailty has been conceptualised using two principal constructs, the Cardiovascular Health Study (CHS) frailty phenotype model, also known as Fried's phenotype (Fried et al. Reference Fried, Tangen, Walston, Newman, Hirsch, Gottdiener, Seeman, Tracy, Kop, Burke and McBurnie2001, Reference Fried, Ferrucci, Darer, Williamson and Anderson2004), and the Canadian Study of Health and Ageing (CSHA) cumulative deficit model, also known as Rockwood's frailty profile (Rockwood and Mitnitski Reference Rockwood and Mitnitski2007). Fried's phenotype (Fried et al. Reference Fried, Tangen, Walston, Newman, Hirsch, Gottdiener, Seeman, Tracy, Kop, Burke and McBurnie2001, Reference Fried, Ferrucci, Darer, Williamson and Anderson2004) describes frailty as a biological syndrome resulting from deficits in five physiological domains: muscle weakness, slowness, exhaustion, low physical activity and unintentional weight loss. A ‘pre-frail’ state is indicated by two of these symptoms, three or more indicating a ‘frail’ state. An alternative construct, the CSHA (Rockwood and Mitnitski Reference Rockwood and Mitnitski2007), proposes that frailty is measured as a risk state in terms of the number of health ‘deficits’ that are manifest in the individual. This model incorporates physical weakness but also polypharmacy, cognition, mental health and activities of daily living constructs. Frailty has been acknowledged as a multi-dimensional concept (Rodríguez-Mañas et al. Reference Rodríguez-Mañas, Féart, Mann, Viña, Chatterji, Chodzko-Zajko, Gonzalez-Colaço Harmand, Bergman, Carcaillon, Nicholson, Scuteri, Sinclair, Pelaez, Van Der Cammen, Beland, Bickenbach, Delamarche, Ferrucci, Fried, Gutiérrez-Robledo, Rockwood, Rodríguez Artalejo, Serviddio and Vega2013; Walston et al. Reference Walston, Hadley, Ferrucci, Guralnik, Newman, Studenski, Ershler, Harris and Fried2006) that includes psychological elements, as well as social elements such as lack of social contacts, situational factors and/or support (Langlois et al. Reference Langlois, Vu, Kergoat, Chassé, Dupuis and Bherer2012; Rodríguez-Mañas et al. Reference Rodríguez-Mañas, Féart, Mann, Viña, Chatterji, Chodzko-Zajko, Gonzalez-Colaço Harmand, Bergman, Carcaillon, Nicholson, Scuteri, Sinclair, Pelaez, Van Der Cammen, Beland, Bickenbach, Delamarche, Ferrucci, Fried, Gutiérrez-Robledo, Rockwood, Rodríguez Artalejo, Serviddio and Vega2013). That is, it is more than just a biological or physiological state.
Physical frailty varies from 4 to 17 per cent in the population aged 65 years and over, while pre-frailty ranges between 19 and 53 per cent in the same age group (Collard et al. Reference Collard, Boter, Schoevers and Voshaar2012). Frail, older adults are high users of informal and formal care and health-care services (Young Reference Young2003), necessitating the developments of prevention and management optimisation in order to enable the sustainability of health-care systems. Frailty can be viewed as a dynamic clinical state, transitioning from robustness through a pre-frail condition to a frail outcome (Ferrucci et al. Reference Ferrucci, Guralnik, Studenski, Fried, Cutler and Walston2004). Although frailty is associated with ageing, it is not inevitable. If identified early, it is suggested that the condition can be halted, reversed, managed and/or prevented (Cameron et al. Reference Cameron, Fairhall, Langron, Lockwood, Monaghan, Aggar, Sherrington, Lord and Kurrle2013; Gill et al. Reference Gill, Baker, Gottschalk, Peduzzi, Allore and Byers2002; Ng et al. Reference Ng, Feng, Nyunt, Feng, Niti, Tan, Chan, Khoo, Chan, Yap and Yap2015; Theou et al. Reference Theou, Stathokostas, Roland, Jakobi, Patterson, Vandervoort and Jones2011).
Screening for frailty is vital for implementing therapeutic measures or interventions. Evidence for the impact of frailty interventions is growing. Successful interventions have included components such as diet and exercise (Theou et al. Reference Theou, Stathokostas, Roland, Jakobi, Patterson, Vandervoort and Jones2011) and may be most effective within the pre-frail ‘intervention window’ (Topinková Reference Topinková2008), although benefits have also been shown for frail people (e.g. Giné-Garriga et al. Reference Giné-Garriga, Guerra, Pagès, Manini, Jiménez and Unnithan2010). To demonstrate effectiveness, interventions should show a real change in key indicators or outcomes of levels of frailty. This would normally include using an explicit operational definition of frailty outlined by authors pre-intervention and followed up post-intervention. Successful interventions should also be targeted at specific barriers to and facilitators of change (Davis et al. Reference Davis, Thomson, Oxman and Haynes1995). Successful interventions in turn reduce the time that older adults’ spend in a frailty or high-dependency state which can significantly improve quality of life and reduce societal health-care costs. Thus, the aim of this survey is to highlight successful aspects of interventions within EIP-AHA partners’ good practices and share their learning experiences.
EIP-AHA
EIP-AHA is a multi-stakeholder collaboration with the aim of increasing the average healthy lifespan in Europe while fostering sustainability of health/social care systems and innovation. In 2013, the EIP-AHA portfolio (EIP-AHA 2013) identified 98 ‘good practices’ involved in research to reverse or prevent frailty. These good practices encompassed six domains: cognitive decline; functional decline; nutrition; care-givers and dependency; physical activity; and general frailty. The aim of this survey is to synthesise and share learning experiences of EIP-AHA partners’ good practices to date.
Method
This survey forms part of a larger study, ‘Frailty Management Optimisation Through EIP-AHA Commitments and Utilisation of Stakeholders Input’ (FOCUS: Cano et al., Reference Cano, Kurpas, Bujnowska-Fedak, Santana, Holland, Marcucci, Gonzalez-Segura, Vollenbroek-Hutten, D'Avanzo, Nobili, Apostolo, Bobrowicz-Campos and Martínez-Arroyo2016), funded by the European Union's Health Programme (2014–20). Ethical approval was provided by the University Research Ethics Committee.
Procedure
Partners were identified through the EIP-AHA portfolio for the A3 Action Group: Prevention of frailty and functional decline (EIP-AHA 2013). The EIP-AHA webpage was searched for additional projects from other action groups, which led to the identification of a further 19 partners. Information from other EIP-AHA groups was examined and four projects relating to falls prevention were included. After identifying duplicates and excluding projects which did not explicitly address aspects of frailty, the survey was emailed to 93 partners. Partners were asked to complete the questionnaire in English using the Bristol Online Survey (University of Bristol 2016) and were assured of anonymity and confidentiality. Five partners replied that their current activities did not match the survey brief and so were removed from the sample. One partner did not wish to participate. Due to low response rates, two follow-up e-mails were sent.
Questionnaire
All participants were given the questionnaire designed to compare their experiences and perceptions of delivering and evaluating good practices. A novel questionnaire was compiled following discussions with the research team and based on work conducted as part of the larger FOCUS project, for example, questions regarding barriers and facilitators to frailty interventions were planned, but also confirmed by qualitative work (Shaw et al. Reference Shaw, Gwyther, Holland, Bujinowska, Kurpas, Cano, Marcucci, Riva and D'Avanzoforthcoming). Partners were asked how they defined frailty, whether they screened for frailty as part of the recruitment and eligibility process, and then whether they measured frailty pre- and/or post-intervention. They were also asked to share details of their intervention. Facilitators, moderators and barriers to intervention success were reported in free-text survey boxes. Facilitators were defined as factors that helped to achieve outcomes that enabled the project or the patients/participants, barriers were defined as factors that stopped achievement of outcomes or that delayed the project or the patients/participants, and moderators were defined as factors that influenced achievement of outcomes or patients/participants (both positively and negatively). According to accepted questionnaire design principles, factual information was collected from partners using categorical questions with a closed range of answers to summarise responses efficiently, while more discursive issues were captured using open-ended responses in free-text boxes to enable partners to give fuller responses and develop a deeper insight into the topic. The full questionnaire can be seen in Appendix 1.
Analysis
Due to the heterogeneity of information provided, only frequency data are reported. An inductive thematic analysis (Braun and Clarke Reference Braun and Clarke2006) of open-ended responses was conducted. Concepts were clustered and synthesised into a final set of themes. All stages of analysis were discussed within the team until consensus was reached.
Results
Characteristics of the partner projects
The sample consisted of 21 partners from seven countries: Greece, Italy, The Netherlands, Poland, Portugal, Spain and the United Kingdom. Three partners chose not to report their country of origin. The final sample (N = 21) reflected a 24 per cent response rate. Table 1 summarises the characteristics of the partners’ good practices.
Table 1. Characteristics of partner interventions, and diversity of frailty screening tools
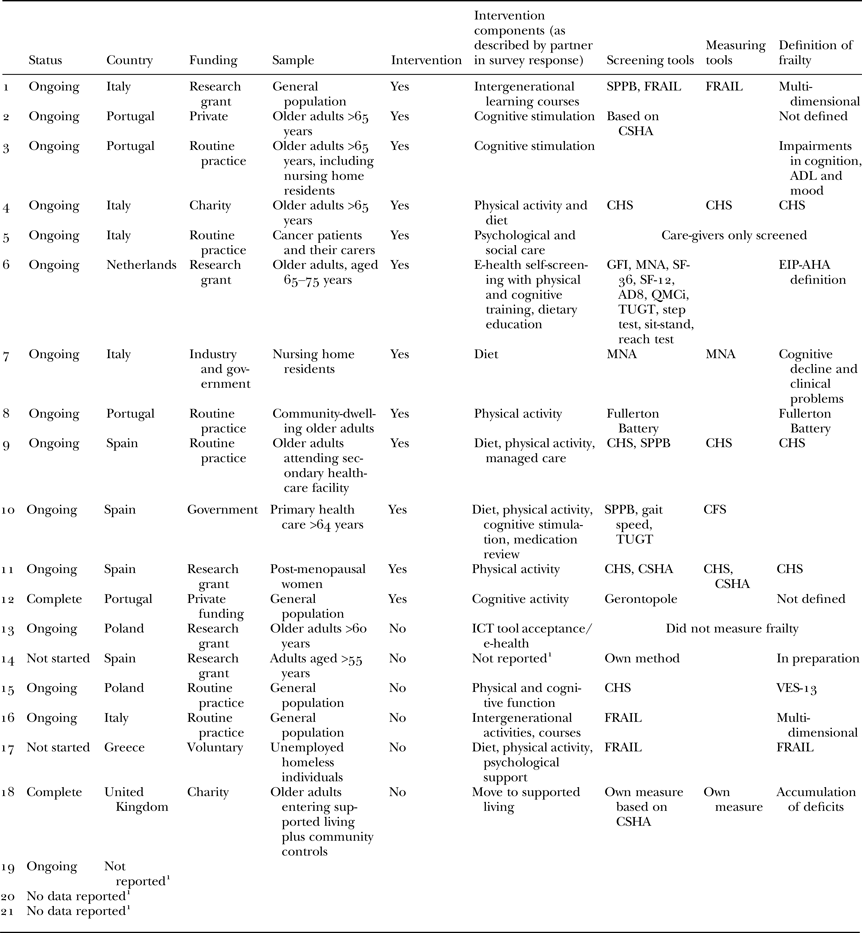
Notes: 1. Although participants did not report these data, they did contribute elsewhere to the study. AD8: Washington University Dementia Screening Test. ADL: activities of daily living. CFS: Clinical Frailty Scale. CHS: Cardiovascular Health Study. CSHA: Canadian Study of Health and Ageing. EIP-AHA: European Innovation Partnership on Active and Healthy Ageing. FRAIL: FRAIL scale. GFI: Groningen Frailty Index. ICT: information and communications technology. MNA: Mini-Nutritional Assessments. QMCi: Quick Mild Cognitive Impairment. SF-12: SF-12 Health Survey. SF-36: SF-36 Health Survey. SPPB: Short Physical Performance Battery. TUGT: Timed Up and Go. VES-13: Vulnerable Elders Survey.
Defining, screening and measuring frailty
The tools used to define, screen and measure frailty varied between good practices (see Table 1). Of the 21 respondents, 13 reported their definitions of frailty, 16 reported general screening for frailty or screening as part of study eligibility requirements, while seven reported measuring frailty status pre- and/or post-intervention. There was no consistent method of screening for or measuring frailty; 61.9 per cent looked for pre-frail individuals or ‘people at risk of becoming frail’. Two studies featured information and communications technology (ICT) or ‘e-health’ interventions.
Of those who defined frailty within their study, most did so in terms of an operational definition, i.e. one that is based on observable criteria and regularly used to identify or diagnose frailty. Six partners chose a relatively narrow definition of frailty based purely on physical health and function: three used CHS criteria (Fried et al. Reference Fried, Tangen, Walston, Newman, Hirsch, Gottdiener, Seeman, Tracy, Kop, Burke and McBurnie2001, Reference Fried, Ferrucci, Darer, Williamson and Anderson2004); one used the Fullerton Battery (Rikli and Jones Reference Rikli and Jones1999, Reference Rikli and Jones2013), one partner reported using the FRAIL scale (Morley, Malmstrom and Miller Reference Morley, Malmstrom and Miller2012) and one partner used the Vulnerable Elders Survey (VES-13). However, a wider approach was used by five partners who adopted a multi-dimensional method based on an accumulation of deficits style or CHSA definition (Rockwood Reference Rockwood2005; Rockwood and Mitnitski Reference Rockwood and Mitnitski2007). Of the remainder, one partner reported that their definition of frailty was still in preparation while the last used the more conceptual definition used by EIP-AHA: ‘Older adults who are at increased risk for future poor clinical outcomes, such as development of disability, dementia, falls, hospitalisation, institutionalisation or increased mortality’ (Fried, Reference Fried, Hazzard, Bierman, Blass, Ettinger and Halter1994).
Of those who reported screening for frailty, either as a general measure or as part of study eligibility criteria, again most used physiological assessments, with some using multiple methods. Four used the CHS phenotype (Fried et al. Reference Fried, Tangen, Walston, Newman, Hirsch, Gottdiener, Seeman, Tracy, Kop, Burke and McBurnie2001, Reference Fried, Ferrucci, Darer, Williamson and Anderson2004), three used the Short Physical Performance Battery (SPPB; Guralnik et al. Reference Guralnik, Simonsick, Ferrucci, Glynn, Berkman, Blazer, Scherr and Wallace1994), three used the FRAIL scale (Morley, Malmstrom and Miller Reference Morley, Malmstrom and Miller2012) and one partner used the Fullerton Battery (Rikli and Jones Reference Rikli and Jones1999, Reference Rikli and Jones2013). Partners also handpicked aspects of physical assessments to suit their methods, e.g. using the Timed Up and Go Test (TUGT) and gait/walking speed calculations. One partner used the Gerontopole (Vellas et al. Reference Vellas, Balardy, Gillette-Guyonnet, Abellan Van Kan, Ghisolfi-Marque, Subra, Bismuth, Oustric and Cesari2013) which is a six-question screening tool primarily based on physical health and functioning but including two questions on cognitive and socio-psychological aspects. Despite five partners defining frailty in a multi-dimensional manner, only three carried this definition forward to their screening and measurement process, using an accumulation of deficits model related to the CHSA approach (Rockwood Reference Rockwood2005; Rockwood and Mitnitski Reference Rockwood and Mitnitski2007). Of these, one described measures of physical and psychological frailty and the other involved a range of instruments including the Groningen Frailty Index (GFI; Schuurmans et al. Reference Schuurmans, Steverink, Lindenberg, Frieswijk and Slaets2004), Mini-Nutritional Assessments (MNA; Guigoz, Vellas and Garry Reference Guigoz, Vellas and Garry1994), AD8, Washington University Dementia Screening Test (Galvin et al. Reference Galvin, Roe, Powlishta, Coats, Muich, Grant, Miller, Storandt and Morris2005), SF-36 Health Survey (Ware, Kosinski and Keller Reference Ware, Kosinski and Keller1994), SF-12 Health Survey (Ware, Kosinski and Keller Reference Ware, Kosinski and Keller1996), Quick Mild Cognitive Impairment screen (QMCi; O'Caoimh et al. Reference O'Caoimh, Gao, McGlade, Healy, Gallagher, Timmons and Molloy2012) and TUGT. One partner assessed nutritional status only via MNA (Guigoz, Vellas and Garry Reference Guigoz, Vellas and Garry1994). Finally, one partner indicated that they would be developing their own screening tool.
Of the seven partners who reported measuring frailty pre- and post-intervention, two used the CHS phenotype (Fried et al. Reference Fried, Tangen, Walston, Newman, Hirsch, Gottdiener, Seeman, Tracy, Kop, Burke and McBurnie2001, Reference Fried, Ferrucci, Darer, Williamson and Anderson2004) alone, and one used it in combination with the CHSA (Rockwood Reference Rockwood2005; Rockwood and Mitnitski Reference Rockwood and Mitnitski2007). Two other partners also used an accumulation of deficit model based on the CHSA (Rockwood Reference Rockwood2005; Rockwood and Mitnitski Reference Rockwood and Mitnitski2007). One partner used the FRAIL scale (Morley, Malmstrom and Miller Reference Morley, Malmstrom and Miller2012) and the last one was solely focused on nutrition measured using the MNA (Guigoz, Vellas and Garry Reference Guigoz, Vellas and Garry1994).
Interventions
Twelve partners reported that their study comprised an intervention (see Table 1). Of these, ten recruited pre-frail individuals. Intervention components addressed aspects of physical performance, cognitive function and directly assessed wellbeing, quality of life, and mental health disorders including depression and anxiety.
Thematic analysis
Facilitators, moderators and barriers
Two themes were identified: working with stakeholders and project management. These were sub-divided into conceptually related sub-themes (see Table 2) which are presented in turn using example data extracts to illustrate their significance. Extracts are attributed by a unique participant identifier, which can be found in Table 1.
Table 2. Numbers of partners identifying facilitators, moderators and barriers to achieving intervention outcomes by theme

Notes: 1. Numbers refer to the total number of partners contributing to each theme or sub-theme. Partners may have contributed more than one data extract to each sub-theme. 2. Numbers refer to each partner's unique identifier. EIP-AHA: European Innovation Partnership on Active and Healthy Ageing. ICT: information and communications technology.
Working with stakeholders
This theme emphasises the need for proactive networks and positive relationships between stakeholders in older adult frailty interventions, in order to build trust, maximise intervention efficacy and prevent communication difficulties. Significant stakeholder relationships described by partners included those between researchers and health/social care professionals; researchers and older adults; health/social care professionals and older adult participants; and within and between older adult participants.
The quality of professional networks and personal relationships were described as critical factors in an intervention's success. Partners described utilising established contacts and existing relationships within their own and stakeholders’ organisations and clinical networks to secure access to participants and gain expertise relevant to the intervention (e.g. nutritional advice). Involving older adult ‘consumers’ and obtaining their point of view was seen as valuable in terms of designing and refining the intervention strategy. Older adult associations, trade unions, geriatric departments, primary and secondary health-care facilities and social services, as well as ‘gatekeeping’ health-care professionals such as general practitioners (GPs) and nurses facilitated participant recruitment. Responses implied that a level of trust and participant knowledge was required for successful recruitment. The data were collected by GPs who know their patients very well (P13). The participation of students, volunteers, health-care professionals and older adults, either as participants or as researchers, was also important in order to actively sustain and administer the intervention:
Without the help of volunteers or students … the success of the intervention would not have been possible. (P6)
However, this reliance on volunteers’ time or busy clinicians’ goodwill to create an effective intervention was a key concern for some partners. Participants described the ‘heavy workload of GPs’ (P13) and the ‘lack of motivation in health professionals’ (P11) as barriers to success. Communication between stakeholders was also cited as a barrier to intervention success. In one instance, this was due to the fragmentation of older adult services and included difficulties relaying information between different organisations and departments, specifically community services, social services and health services:
Isn't always easy have an effective communication in order to guarantee the best services. (P5)
Connexion between community services, social services and health services. (P10)
Further, there were issues where communication difficulties and misunderstandings among health and social care professionals regarding participant eligibility created difficulties in obtaining a representative sample:
Some issues with nurses at the beginning only sending us the healthier active people to the project – we sorted it! (P18)
While procedures and strategies could be put into place in order to recruit participants, retaining participants was viewed by partners as more demanding. Programmes which incorporated an element of perceived benefit to older adults reported success in terms of participant adherence, specifically, interventions which included a social element, an ‘opportunity to socialise’ (P4) either with peers or intergenerationally; or those that provided health advice.
The adherence to the programme was remarkable; it … create [sic] an environment where people have fun, learn and where they strengthen abilities and relationships. (P3)
Other factors such as ensuring that interventions occurred in geographically convenient and physically accessible environments were also raised:
The fact that we went to them was crucial, rather than expecting them to come anywhere. (P18)
Being conducted in primary care, with the participants’ reference doctors and nurses involved and interventions close to their homes with no waiting lists. (P10)
Partners also described cultural and social factors that moderated achievement of intervention outcomes including: increasing age, with the ‘young’ old functioning better in interventions; lower socio-economic status with fewer representatives from lower socio-economic classes; and gender imbalances with women outnumbering men and becoming more involved in social activities. Those who had a higher cognitive capacity, were better educated, or who had previous experiences in volunteering or third sector activities were more likely to be represented and consequently successful. Barriers to intervention success also included participants being unwilling or unable to participate due to lack of interest, lack of time and social support, family commitments or personal issues, e.g. unemployment, bereavement and ill-health (including depression). Partners also described cultural barriers for older adults in terms of information technology literacy and difficulties using ICT tools and preferences for face-to-face/personal contact over online methods. There were also cultural differences in intervention success between countries, regions and communities based on the support of the church (which appeared to improve recruitment and adhesion) and the support of government (which was not always uniform or strategic across regions). In one instance, a partner called for reform in health policy strategy in order to stimulate integration between public health care and social care enterprises.
In short, this theme describes the stakeholder commitment required to generate a successful intervention. Building and maintaining effective relationships and interactions between stakeholders is crucial in ensuring success, whether they are researcher–participant; researcher–health/social care professional; health/social care professional–older adult; or older adult–older adult. It was also clear from partners’ accounts that social engagement was a contributing factor to intervention success which could help researchers to improve their recruitment and retention statistics and positively benefit older adults. The next theme turns towards the project management and administrative aspects of the intervention to explore the most important facilitators, moderators and barriers of intervention success.
Project management
Many partners acknowledged the difficulties of appropriately resourcing projects in the current fiscal environment of austerity. The engagement and support of institutions such as public health authorities or municipalities were cited as factors critical to success, either as a means of providing resources, structure, logistics or official permissions (ethics). Conversely, limited support by governing bodies, e.g. institutions, public institutions and EIP-AHA, as well as bureaucracy, were said to be hindrances. For partners, access to adequate financial resources was integral to success; a lack of funding meant that good practices struggled to deliver. Given that many of the projects were undertaken voluntarily, or within the scope of normal clinical duties, bureaucracy was viewed as a major burden and source of frustration by partners. One participant expressed significant tensions in their relationship with EIP-AHA:
No funding or support from EIP-AHA, only repeated requests for updates etc. Gets really tiresome to be honest. Tired of repeatedly going to Brussels and giving updates and no support. (P19)
This partner described excessive meeting commitments without reciprocal financial support, an opinion which was supported by others. This meant that teams were diverted from their primary task of intervention delivery, which in turn affected patient/participant outcomes.
Partners described how leadership at different organisational levels was important in managing an intervention, both at local site level and higher institutional levels. Some partners described well-functioning research networks characterised by leaders who supported the intervention and were fully engaged with it. Ownership and autonomy were critical factors, with an example of managers taking responsibility for the intervention through involvement in the development of the good practice:
The different partners have the autonomy to manage the process, as they have participated in the development and validation of the good practice. (P3)
Further, EIP-AHA partners expressed how management support was critical in ensuring the co-operation of staff responsible for promoting and implementing the intervention:
On site well-being nurses supported recruitment and management encouraged them to do so. (P18)
In this instance, nurses were able to facilitate participant recruitment because the management were engaged in, and supportive of, the intervention process. Conversely, ‘scarce institutional support’ (P16), presumably a failure of management to demonstrate appropriate support, was cited as a barrier to intervention success and led to a ‘reluctance to innovative practices’ (P11). This notion of engaged and active leadership was significant in facilitating the intervention and encouraging staff to promote and engage in sometimes novel practices.
This theme presents the challenges to intervention success from resourcing constraints, excessive bureaucracy and disengaged leaders, along with the positive opportunities that arise for intervention participants when institutions, managers and individuals work together.
Discussion
The aim of this survey was to synthesise and share learning experiences of partners within the EIP-AHA frailty projects. Despite considerable variation in the sample types, settings and study designs making direct comparisons difficult, the results are sufficiently cohesive to provide suggestions for improving intervention success.
The effectiveness of an intervention relies on prompt screening and identification of older people at risk of frailty. Of the 12 partners delivering interventions, two did not identify pre-frail individuals. Given that frailty interventions are likely to be most effective in the ‘pre-frail’ intervention window (Topinková Reference Topinková2008), this may represent a missed opportunity for a successful health-care intervention. The emphasis on pre-frail participants, however, may also indicate that partners perceived more significant levels of frailty as less amenable to intervention, whereas, in fact, evidence suggests that even very frail people can benefit from intervention (e.g. Giné-Garriga et al. Reference Giné-Garriga, Guerra, Pagès, Manini, Jiménez and Unnithan2010).
The operational definitions of frailty, methods, and rates of screening for and measuring frailty varied between partners. Twelve different methods were used with CHS (Fried et al. Reference Fried, Tangen, Walston, Newman, Hirsch, Gottdiener, Seeman, Tracy, Kop, Burke and McBurnie2001) and CHSA models (Rockwood Reference Rockwood2005) the most utilised. Further, some researchers were developing their own strategies. Although strict definitions and measurement have been a challenge in frailty research, there are a selection of valid, reliable and diagnostically accurate tools for assessing frailty (Pijpers et al. Reference Pijpers, Ferreira, Stehouwer and Nieuwenhuijzen Kruseman2012), as well as simple risk indicators such as gait speed (Apostolo et al. Reference Apostolo, Cooke, Bobrowicz-Campos, Santana, Marcucci, Cano, Vollenbroek-Hutten and Holland2015), and these should be used widely to enable comparisons. Researchers should be discouraged from developing their own measures unless they have the resources necessary to establish the reliability and validity of these measures. These findings support a recent umbrella review of the reliability and validity of frailty screening tools in which the authors called for a method consensus on frailty and/or pre-frailty screening (Apostolo et al. Reference Apostolo, Cooke, Bobrowicz-Campos, Santana, Marcucci, Cano, Vollenbroek-Hutten and Holland2015; Apostolo et al. Reference Apóstolo, Cooke, Bobrowicz-Campos, Santana, Marcucci, Cano, Vollenbroek-Hutten, Germini and Hollandin press). Similarly, pre- and post-intervention frailty evaluations should be properly designed, with valid and reliable measures which are consistently applied.
Systematic development of interventions can also be achieved through proper design or intervention mapping, tailoring content and format to specific features, contexts and needs of a target group (Davis et al. Reference Davis, Thomson, Oxman and Haynes1995). The findings regarding specific barriers to and facilitators of intervention success could facilitate future intervention success and improve their effectiveness.
Recruitment techniques and study adherence are critical in intervention success as under-representation of older adults may lead to inaccurate conclusions being drawn about intervention effectiveness. Within the qualitative data, there was a strong theme of study adherence through social participation and geographical convenience. The studies describing strong rates of adherence occurred in the local community. They incorporated an element of perceived benefit to older adults that was viewed positively, either a social aspect or health-care promotion. Although social exchange can be time consuming and resource intensive, in this study, it appeared to be a strong facilitator of success and may be seen as a ‘reward’ for isolated older adults (Provencher et al. Reference Provencher, Mortenson, Tanguay-Garneau, Bélanger and Dagenais2014). In addition, these projects were geographically and physically convenient and accessible. Ensuring convenience is a way to compensate for older adults’ health or mobility problems which may make it difficult for them to travel (Provencher et al. Reference Provencher, Mortenson, Tanguay-Garneau, Bélanger and Dagenais2014). The findings support previous work that older adult recruitment and participation is affected by a number of factors including ‘research burden’, life events, availability of transport and trust (Areán and Gallagher-Thompson Reference Areán and Gallagher-Thompson1996).
Another factor for older adults was distrust and fear of researchers (Provencher et al. Reference Provencher, Mortenson, Tanguay-Garneau, Bélanger and Dagenais2014). One successful method of overcoming this was through the establishment of research partnerships with more trusted professionals (e.g. GPs, geriatric clinics and trade unions). These gatekeepers were reported as being significant in arbitrating access to older adults. However, care must be taken to ensure that gatekeepers do not over-censor, thereby excluding potentially suitable candidates. Further, partners reported that a lack of project funding placed an additional administrative burden on professional volunteers who already have substantial workloads.
Partners criticised the EIP-AHA model as being overly bureaucratic and insufficiently financially supportive. Some partners were undertaking interventions as part of their normal clinical duties without additional funding and to an extent these had developed organically with post-hoc evaluations occurring retrospectively. A review of administrative requirements would be beneficial, with facilitation of prospective and fully planned evaluations.
Leadership was another factor in intervention success and determining staff commitment. Strong, supportive leadership across all aspects of the intervention was described, with leadership filtering down from an organisational/institutional level with commitment, study approval and provision of appropriate resources, to a local level whereby clear direction, communication, support, enthusiasm and encouragement appeared to enable staff delivery. The link between leadership support, vision and regulatory factors has been influential in other studies, e.g. facilitating the transfer of research findings into clinical practice in health-care professionals (Gifford et al. Reference Gifford, Davies, Edwards, Griffin and Lybanon2007).
One limitation of this research is that only participants who responded to the invitation to take part were included. The EIP-AHA portfolio includes a broad range of frailty projects, many of which do not involve human participants, e.g. some involve basic research, epidemiology and animal modelling. Of the invited 93 EIP-AHA portfolio projects (EIP-AHA 2013), only 34 specifically address frailty in human participants, suggesting that this was a reasonable response rate. Other potential explanations for the response rate may be: that projects reported in the EIP-AHA portfolio (EIP-AHA 2013) have been completed and researchers have moved on; that projects have not started or have not reached the intervention phase and so there is limited information to report; or there may be an unwillingness to take part due to the high levels of bureaucracy placed on EIP-AHA participants. Irrespective, the survey reported information from 21 partners involved in frailty research or frailty interventions in seven European countries. While there were notable absences from Northern Europe, specifically Scandinavia, France and Germany, projects in Southern Europe were better represented. The distribution of projects is in line with findings from the EIP-AHA portfolio (EIP-AHA 2013).
Conclusion
Early diagnosis can help improve care for older adults, helping to prevent the progression of frailty and potentially reduce societal health and social care costs. These findings describe the outcome of a survey conducted within the framework of a European Union-funded project aimed at reducing the burden of frailty within Europe. Although the response rate was modest, results illustrate the potential to standardise frailty interventions in terms of increasing the proportion of projects that screen, assess or measure for frailty using consistently applied reliable and validated methods as well as providing valuable information for intervention mapping in terms of barriers to and facilitators of frailty interventions.
Acknowledgements
This work was supported by the Consumers, Health, Agriculture and Food Executive Agency (CHAFEA) of the European Commission, under the European Union Health Programme (2014–20). The survey forms part of a larger study, ‘Frailty Management Optimisation Through EIP-AHA Commitments and Utilisation of Stakeholders Input’ (grant number 664367 FOCUS). We acknowledge the contribution of other members of the FOCUS project: A. Nobili (IRCCS Istituto Di RicercheFarmacologiche “Mario Negri”, Italy), A. González Segura (EVERIS Spain S.L.U, Spain), A. M. Martinez-Arroyo (ESAM Tecnología S.L., Spain), B. D'Avanza (IRCCS Istituto Di Ricerche Farmacologiche “Mario Negri”, Italy), D. Kurpas (Wroclaw Medical University, Poland), E. Bobrowicz-Campos (Nursing School of Coimbra, Portugal), E-A, Jaime Dauden (University of Valencia, Spain), F. Germini (IRCCS Ca'Grande Maggiore Policlinico Hospital Foundation, Milan, Italy), J. Apostolo (Nursing School of Coimbra, Portugal), L. van Velsen (Roessingh Research and Development, Netherlands), M. Bujnowska-Fedak (Wroclaw Medical University, Poland), S. Santana (University of Aveiro, Portugal), T. Kujawa (Wroclaw Medical University, Poland), who were co-responsible for the design and delivery of this FOCUS work package. Ethical approval was provided by Aston University Research Ethics Committee (#887).
Appendix 1
Please type the name of the good practice here:
Please type the name of country in which the good practice is located here:
1) Definitions and measures of frailty
Did you screen for or measure frailty in your good practice?
Screened for frailty □ Measured frailty □ Neither screened nor measured frailty □
Other please specify ____________________
How did you define frailty in your good practice?
__________________________________________________________
__________________________________________________________
How did you measure frailty in your good practice?
__________________________________________________________
__________________________________________________________
2) Screening methods and strategies
How many people did you plan to screen?
<100 100–1000 >1000 Not applicable (if you tick this option please go to 3)
How many people did you screen?
<100 100–1000 >1000
If you screened for frailty, was this
in a specified group (please state) ______________________________
general population screening __________________________________
other (please state) ___________________________________________
3) Identification methods
What methods did you use to identify individuals at risk of frailty
Specific standardised cut-off criteria or values? (please state)________
Specific diagnoses of frailty (please state)_________________________
Specific care needs/uses? (please state)__________________________
Other methods (please state)___________________________________
Did not identify individuals in terms of frailty (please outline how you selected your participants (i.e., what inclusion/exclusion criteria did you use?)
____________________________________________________________
____________________________________________________________
4) Intervention categories
Was your good practice an intervention? Yes No
If yes, please tick (check) the design that best describes your project:
a. RCT [randomised control trial]
b. Observational study
c. Non-randomised control trial
d. Case control study
e. Other (please state)_______________________________________
If no, please describe your design here:
______________________________________________________________
______________________________________________________________
What intervention categories were included in the good practice? (examples here would be diet, physical activity, etc.)
______________________________________________________________
______________________________________________________________
How many people did you plan to reach with your intervention?
<100 100–1000 >1000 Not applicable
How many people did you reach with your intervention?
<100 100–1000 >1000 Not applicable
5) Outcome measures
Please report the outcome measures you used in your good practice
____________________________________________________________
____________________________________________________________
6) Facilitators to achieving outcomes for patients
Please outline the MAIN organisational facilitators to achieving outcomes for patients (e.g., factors that helped to achieve outcomes that enabled the project or the patients/participants)
____________________________________________________________
____________________________________________________________
Did any organisational factors (e.g., organisational culture, governmental support) moderate the achievement of outcomes for patients?
____________________________________________________________
____________________________________________________________
Please outline the MAIN patient/participant facilitators to achieving outcomes for patients (e.g., factors that helped to achieve outcomes that enabled the project or the patients/participants)
____________________________________________________________
____________________________________________________________
Did any patient/participant factors (e.g., age, gender, ethnicity) moderate the achievement of outcomes for patients?
____________________________________________________________
____________________________________________________________
7) Barriers to achieving outcomes for patients
Please outline the MAIN organisational barriers to achieving outcomes for patients (e.g., factors that stopped achievement of outcomes that delayed the project or the patients/participants)
____________________________________________________________
____________________________________________________________
Please outline the MAIN patient/participant barriers to achieving outcomes for patients (e.g., factors that stopped achievement of outcomes that delayed the project or the patients/participants)
____________________________________________________________
____________________________________________________________
8) Evaluation criteria
What evaluation criteria did you use to assess the impact of your good practice on outcomes that are important to patients?
____________________________________________________________
____________________________________________________________
What evaluation criteria did you use to assess the impact of your good practice on economic outcomes?
____________________________________________________________
____________________________________________________________
9) Internal/external monitoring of good practice
Was there any internal monitoring of the activities in your good practice (e.g., steering group/committee, team meetings, review meetings, away days)?
____________________________________________________________
____________________________________________________________
Was there any external monitoring of the activities in your good practice (e.g., steering group/committee, quality assessment teams, funder meetings)?
____________________________________________________________
____________________________________________________________
10) Type of professionals involved
Please list the different professionals (e.g., clinicians, nurses, healthcare assistants, researchers, etc.) involved in the delivery of your good practice
____________________________________________________________
____________________________________________________________
Please describe the composition of the team involved in delivering your good practice (i.e., a team of 5 nurses, 2 consultants, etc.)
____________________________________________________________
____________________________________________________________
11) Type of health system
Can you describe, in your own words, the type of health system you work in (i.e., government/publicly funded, private funding, charity funding)
____________________________________________________________
____________________________________________________________
12) What is the current status of your good practice?
Completed Ongoing Not started yet
13) How is your good practice funded?
a. Research grants
b. Government funds
c. Routine practice (no additional economic support)
d. Industry funding
e. Other (please describe)
____________________________________________________________
____________________________________________________________
14) Data sharing
One of the aims of the FOCUS project is to promote a scaling-up process of A3 Good Practices through their analytical comparison upon process and outcome indicators. Would you be willing to share either aggregate results or individual data from your good practice with the FOCUS project team?
I WOULD BE WILLING TO SHARE
a. Aggregate data with the project team
b. Individual data with the project team
c. I would not be prepared to share data with the project team




