Autism-spectrum disorders are one of the leading causes of lifetime developmental disability (Reference FombonneFombonne 2003). These diagnoses are often grouped as pervasive developmental disorders and include childhood autism, atypical autism and Asperger syndrome. They are recognised as complex neurodevelopmental disorders, often becoming clinically apparent in the second to third year of life. The diagnosis is based on disturbance in the domains of: reciprocal social interactions, communication, restricted interests and stereotyped patterns of behaviour (World Health Organization 1994). Up to two-thirds of affected individuals will present with a degree of global intellectual disability, although some may have a very uneven profile of abilities. Accurate diagnosis, usually made by a combination of direct observation of behaviour and informant history, is complicated by considerable heterogeneity in the manifestation of these core deficits, by variation in ability level and by developmental changes. However, it is clear that autism-spectrum disorders persist across the lifespan, with varied complex needs in adult life (Reference Lord, Leventhal and CookLord 2001). The course of development into old age is, as yet, largely unknown.
Prevalence figures vary widely depending on the definition of caseness and exact assessment tools used. Until recently, prevalence figures of 0.3–0.9% were reported for all autism-spectrum disorders (Reference FombonneFombonne 2005). Latterly, UK studies have reported a higher prevalence of around 1.2%, which probably reflects better ascertainment of a broader phenotype (Reference Baird, Simonoff and PicklesBaird 2006). There are no accurate prevalence figures for adults with autism. A male excess of between 3:1 and 4:1 is generally observed (Reference FombonneFombonne 2003).
Maladaptive behaviours and comorbid psychiatric symptoms are common in individuals with autism-spectrum disorders and are strongly associated with caregiver stress (Reference Lecavalier, Leone and WiltzLecavalier 2006).
Autism is thus a relatively common, chronic, potentially substantially disabling disorder, with significant costs to both the affected individual and to family members. There are no established definitive treatments for the core social impairment. Both the severity of the disorder and lack of effective treatments continue to promote keen public interest and prompt research. Controversies surrounding environmental causes of autism and novel unproven treatments serve to keep this set of disorders in the public eye and increase referrals to both paediatric and psychiatric services. Since the introduction of the National Autism Plan for Children in the UK (National Initiative for Autism: Screening and Assessment 2003), there has been a further focus on early intervention as well as assessment. More people are now first diagnosed with autism as adolescents and adults, possibly owing to increased public and professional recognition (Reference Mesibov, Handlan, Cohen and VolkmanMesibov 1997). For many families, the transition from adolescence to adulthood will be a period of increased need. As a result, a greater number of referrals may be seen across adult psychiatry.
Medication management in autism
Alongside burgeoning professional interest, increasingly rigorous clinical trial methodology has attempted to focus on evidence-based pharmacological strategies for both the core syndrome and comorbidity in autism-spectrum disorders. In the field of neurodevelopmental disability there has traditionally been a dearth of independent, well-powered randomised controlled trials (RCTs). However, more rigorous psychopharmacology studies have recently started to be published. Educational and psychosocial interventions are the mainstay of treatment, with the aim of improving language acquisition and maximising communication and social skills.Footnote † However, medication management, particularly of maladaptive behaviours, is common and possibly increasing (Reference Aman, Lam and van BourgondienAman 2005). Such behaviours or symptoms of comorbid disorders may interfere with socialisation and educational progress, and severely impair individuals' and families' quality of life. Up to 75% of individuals with autism and intellectual disabilities were prescribed at least one psychotropic medication in a recent UK study (Reference Tsakanikos, Costello and HoltTsakanikos 2006). Factors associated with increasing medication use include greater age, poorer functioning and higher levels of challenging behaviour (Reference Aman, Lam and van BourgondienAman 2005).
There are, however, no currently available standard medication regimes. Medications that are commonly used belong to diverse groups and are non-specific to the target symptoms identified. They may affect a wide range of neurological functions, with subsequent unwanted effects. An attempt to elucidate the evidence base for the use of such treatments is therefore helpful for prescribers and case managers alike.
The aim of this paper is to discuss and summarise clinical pharmacotherapy for autism-spectrum disorders based upon target domains of behaviour (Table 1). Such symptoms, which are difficult to manage, are often the reason that families seek medical treatments.
We first evaluate treatments targeted at the underlying core social deficit of autism and then address certain target clusters of symptoms such as stereotypical and compulsive/ritualistic behaviours, and serious aggressive and self-injurious behaviour. The distinction between core syndrome phenomena and co-occuring symptoms is important in assessment and treatment planning, even though this distinction is sometimes rather theoretical: improvement in one area may lead to global improvements, and some drugs may play a role in several symptom clusters. However, a systematic approach that makes this distinction will help clinicians organise their thinking when deciding on a particular medication strategy, allowing them to focus on exactly which presenting problem they are trying to treat, and with what. A methodical approach of this kind is also essential to minimise polypharmacy and the development of potentially disastrous management strategies in which further medication has to be used to treat emerging unwanted effects of previous medications.
We will focus on RCTs, where available, as well as common unwanted effects of particular drugs and shortcomings of the published data. Although the field is not yet at a stage at which treatment protocols or algorithms could usefully be written, the aim will be to identify medication regimes which are most likely to be clinically useful for each domain. Medication management of common psychiatric comorbidities in autism will also be discussed.
Finally, future directions of pharmacotherapeutic research will be highlighted.
Core autism-spectrum disorder symptomatology
Social deficits
The detailed underlying pathophysiology of social impairments in autism-spectrum disorders is still unclear. Their treatment has been subject to many ‘false dawns’ in the literature, with therapeutic approaches often based on questionable theory. Some rigorous trials have on occasion reduced interest in unproven approaches despite their active promotion by vested interests. Most recently, secretin, a gastrointestinal peptide, and fenfluramine, an indirect 5-HT partial agonist, have been widely trialled, with nearly 500 children with autism-spectrum disorders being recruited to RCTs for secretin alone. Both drugs were shown in preliminary trials to have prosocial effects in autism. However, placebo-controlled trials have failed to show consistent improvements (Reference McDougle, Stigler and EricksonMcDougle 2006). Neither medication has any evidence base in autism management at this time.
Diet
Vitamins and minerals have been widely trialled, with broadly disappointing results, a recent example being a Cochrane review of RCTs studying the use of vitamin B6 and magnesium (Reference Nye and BriceNye 2005). It was concluded that their use cannot be supported in autism-spectrum disorders based on the available evidence, and that larger, better designed studies are needed. There is widespread interest in exclusion diets, but as yet there have been no good-quality RCTs in this area.
Immune function
Infectious and immune mechanisms have been popular candidate aetiological agents in autism (although there is no significant support for this from basic science). A limited number of related treatment studies have been carried out. One trial of vancomycin (Reference Sandler, Finegold and BolteSandler 2000) showed that improvements in communication returned to baseline when the drug was stopped. Systematic RCTs of drugs having a direct effect upon immune function in autism have not so far been conducted.
There is, at present, no place for immunotherapy of any kind in the management of autism. There is also no reliable evidence that anti-fungal treatments are effective in the treatment of autism-spectrum disorders.
Vaccinations, particularly the combined measles, mumps and rubella (MMR) vaccine, have long been the subject of controversy with regard to aetiological mechanisms and autism-spectrum disorders. Several large-scale studies have subsequently not supported a causal link between MMR and autism (Reference Honda, Shimizu and RutterHonda 2005).
Antidepressants and antipsychotics
Open-label reports of selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics such as risperidone have suggested that some individuals with autism show improvement in aspects of social relatedness following treatment (Reference McDougle, Stigler and EricksonMcDougle 2006). This has yet to be consistently established in RCTs (Reference Hollander, Phillips and ChaplinHollander 2005), but some clinicians would support their empirical use. Risperidone does have a proven effect on arousal states and behaviour (see below), and any effect on prosocial behaviour is likely to be secondary to this.
Glutamate-active medication
Glutamatergic function has been the focus of recent extensive research in neuropsychiatric disorders. Glutamate is the primary excitatory amino acid in the brain and is thought to be important in regulating neuronal plasticity and higher cognitive functions (Reference CarlssonCarlsson 1998). Both autism-spectrum disorders and schizophrenia are postulated to be hypoglutamatergic disorders and parallels have been drawn between the negative symptoms of schizophrenia and the social impairment in autism (Reference Nikolov, Jonker and ScahillNikolov 2006).
Further interest has been excited by a recent large pooled genetic analysis (Autism Genome Project Consortium 2007) which used linkage and copy number variation analysis respectively to implicate candidate gene loci on chromosome 11p12-p13 and neurexins. Neurexins and neuroligins (independently linked with autism in other analyses) are implicated in glutamatergic synaptogenesis, highlighting glutamate-related genes as promising candidates in autism-spectrum disorders. One glutamate-active drug of interest is d-cycloserine (a partial agonist at the n-methyl-d-aspartic acid (NMDA) receptor complex). It has been used with benefit when added to conventional antipsychotics in schizophrenia and this benefit has been associated with enhanced temporal lobe function (Reference Yurgelun-Todd, Coyle and GruberYurgelun-Todd 2005). A small single-blind pilot study of d-cycloserine targeting the core social impairment in children with autism-spectrum disorders (Reference Posey, Kem and SwiezyPosey 2004) showed greater improvements on the Clinical Global Impressions (CGI) scale and the Aberrant Behaviour Checklist (ABC) social withdrawal scale, with ascending dosages. Adverse effects were reported only at higher doses. However, Posey recently reported a larger, double-blind parallel groups study which showed that d-cycloserine did not improve social withdrawal in young children (Reference PoseyPosey 2008). Additional subgroup analyses are planned to determine whether individual characteristics are associated with response. Other drugs currently being trialled include amantadine and memantine, which act as non-competitive antagonists at the NMDA receptor.
One small controlled trial of amantadine in children (Reference King, Wright and HandenKing 2001) showed a trend towards greater treatment response based on CGI ratings. Other studies have been less optimistic, for example lamotrigine (an anticonvulsant which attenuates cortical glutamate release) was found to be no better than placebo on any outcome measure employed in a small RCT in children (Reference Belsito, Law and KirkBelsito 2001). The active research interest in this area parallels that in schizophrenia and more work in this area will undoubtedly follow. However, the work is at a preliminary stage and at present the available evidence would not support the clinical use of glutamate-active medications in autism.
Clinical implications
The core social impairments in autism-spectrum disorders remain relatively intractable. In childhood and adolescence, psychosocial and education management strategies should be the first line in management and do have an evidence base for efficacy in some situations. The current lack of convincing evidence for a pharmacological approach to long-term disabilities means that at present medication management does not have a significant place in practice. Future advances may change the situation.
Stereotypical and ritualistic/compulsive behaviours
These behaviours are defined as part of the core of autism. They include verbal and motor rituals, obsessive questioning, rigid adherence to routine, preoccupation with details and obsessive desire for maintenance of sameness. Such symptoms are commonly the most functionally impairing aspect of the syndrome and interfere significantly with an individual's progression in educational and socialisation programmes. They have similarities with obsessive–compulsive disorder (OCD) phenomena and thus medications such as tricyclic antidepressants and SSRIs with known efficacy in OCD are obvious candidate drugs.
Antidepressants
Clomipramine is a non-selective tricyclic antidepressant which affects uptake of serotonin, noradrenaline and dopamine. Two crossover RCTs using children and young adults provide some evidence that clomipramine is superior to placebo on measures such as anger and obsessive symptoms. However, there were no gains for areas such as hyperactivity and global symptom severity (Reference Remington, Sloman and KonstantareasRemington 2001). Concerns about adverse effects of clomipramine make this drug less widely used than SSRIs.
Using the SSRI fluvoxamine, a short-term RCT in adults with autism showed improvements in both compulsive and prosocial behaviour (Reference McDougle, Naylor and CohenMcDougle 1996). Trials with children have had more mixed results. A short-term RCT involving children with autism-spectrum disorders showed that fluvoxamine had no advantage over placebo (Reference McDougle, Kresch and PoseyMcDougle 2000). Adverse effects were noted in 78%. More encouragingly, another 12-week RCT crossover study of 18 children with autism-spectrum disorders judged that 10 were treatment responders on the CGI. However, a placebo response rate is not recorded, and adverse events occurred in 39%, although this was not considered significant (Reference Sugie, Sugie and FukudaSugie 2005).
Improvements in repetitive behaviours have been shown in two very small crossover RCTs in children and adults using fluoxetine (Reference Buchsbaum, Hollander and HaznedarBuchsbaum 2001). There were no differences in side-effects between drug and placebo in children (Reference Hollander, Phillips and ChaplinHollander 2005). Unpublished data of a recent large-scale RCT of fluoxetine in children showed that repetitive behaviours were reduced in those taking either placebo or fluoxetine but there were no statistically significant differences in response between the two groups. Full analysis of the trial is ongoing to establish the reasons for this unexpected result (Autism Speaks 2009).
Evidence regarding other antidepressant use is scant and of less quality. Open-label trials of sertraline, citalopram and escitalopram in children and adults have showed benefits in reducing aggression and repetitive behaviours (e.g. Reference Owley, Walton and SaltOwley 2005). An open-label study using paroxetine in adults showed improvements at 1-month but not at 4-month follow-up (Reference Davanzo, Belin and WidawskiDavanzo 1998). There are no studies using paroxetine in children as yet. A recent large-scale RCT of citalopram in children with autism-spectrum disorders and repetitive behaviours showed no significant differences in response between the citalopram- and placebo-treated group, although both groups showed improvements. Citalopram was more likely to be associated with adverse events such as increased energy levels and insomnia (Reference King, Hollander and SikichKing 2009). Open-label studies of venlafaxine have suggested efficacy in repetitive behaviours in some children and adults (Reference Hollander, Kaplan and CartwrightHollander 2000).
The studies above may indicate that SSRIs may be more effective and give rise to fewer side-effects in adolescents and adults with autism-spectrum disorders than in children (Reference Erickson, Posey and StiglerErickson 2007).
Antipsychotics
Two large multisite trials in children (Reference Shea, Turgay and CarrollShea 2004; Reference McDougle, Scahill and AmanMcDougle 2005) showed that risperidone was significantly more effective than placebo for reducing interfering behaviours. However, in smaller RCTs with preschool age children with autism, risperidone was not meaningfully superior to placebo (Reference Luby, Mrakotsky and StaletsLuby 2006). Adverse effects of atypical antipsychotics include increased appetite and weight gain, dyslipidaemia and insulin resistance, somnolence, extrapyramidal symptoms and prolactin elevation. One study looking at prescribing of risperidone over the course of 12 months used a pooled database of prolactin levels in 700 children (Reference Dunbar, Kusumakar and DanemanDunbar 2004). Mean levels were found to increase and peak in the first 1–2 months and then return to near normal by 3–5 months. There was no associated delay in growth or sexual maturation.
Prolongation of the QTc interval with risperidone has been reported, but studies in both adults and children did not find prolongation beyond the threshold accepted as being associated with torsade de pointes or other significant electrocardiogram (ECG) changes (e.g. Reference Harrigan, Miceli and AnzianoHarrigan 2004).
Clinical implications
In this domain of core autism symptomatology, the situation is rather the reverse of the social deficits. Psychosocial or cognitive–behavioural interventions can be useful adjuncts but may be less effective in this patient group than they can be, for example, in people with autism and comorbid obsessive–compulsive symptoms. Medication management has some evidence base and clinical experience suggests it can be useful. Medication effects, however, do show a great deal of individual variation, and patients and families need to understand that trials of medication may be exploratory and be subject to careful monitoring and dosage adjustment. A recent review (Reference McDougle, Stigler and EricksonMcDougle 2006) suggests that, given the side-effect profile of risperidone, low-dose SSRIs should be used as first-line treatment if repetitive behaviour is the main focus of treatment. However, even the evidence base for SSRIs consists of small short-term RCTs with heterogenous populations and varying outcome measures.
Co-occurring or comorbid psychiatric symptoms
Autism-spectrum disorders have a high prevalence of both psychiatric and physical comorbidities (Reference Broadstock, Doughty and EgglestonBroadstock 2007). These tend to emerge in middle childhood, and wax and wane according to circumstance. At a theoretical level it is not always clear that such symptoms constitute a true psychiatric comorbidity; commonly, symptoms will co-occur with autism because of the interaction between the developmental disorder and concurrent stressors such as increased social demands, inadequately adapted schooling, a sense of rejection or poor self-image, bullying or family conflict. These symptoms are important to understand since they are often as functionally impairing and yet more tractable than the core disorder, and are often a key target for intervention.
Accurate diagnosis of comorbid psychiatric disorders can be difficult. Modifications of diagnostic criteria may be necessary to account for differing clinical presentations in individuals with developmental disability. ‘Diagnostic overshadowing’ may prevent accurate detection of symptoms; even if detected, they may be spuriously attributed to the core disorder (Reference DykensDykens 2000). It is also clear, however, that a condition like autism will be associated with additional symptoms which may not rise to the level of ‘disorder’. Deciding when and whom to treat may be largely based on functional impairment. Conditions such as depression and anxiety (as opposed to the core syndrome) may be eminently treatable and tackling them may vastly improve patients' and families' quality of life.
A growing body of literature is focused on the evidence base for treating symptoms of hyperkinetic disorder in autism. However, there is a relative dearth of high-quality data considering other psychiatric comorbidities. Most of the available data come from trials designed to assess core and behavioural symptoms associated with autism, but which also found improvements in other domains such as depressive or anxious symptoms. Randomised controlled trials studying patients with both autism-spectrum disorders and adequately defined psychiatric comorbidities are urgently needed, but are difficult to design and carry out. Much of the data on psychiatric comorbidity in adolescents and adults are based on case reports and are difficult to interpret given the potential for selection, referral or reporting biases.
Maladaptive aggression and self-injury
Self-injurious behaviour and aggression can severely disrupt the management of autism. Many pharmacological treatments have been trialled.
Antipsychotics
Atypical antipsychotics are the most frequently used psychotropic medication for aggression and serious self-injury in people with autism (Reference Erickson, Posey and StiglerErickson 2007). Moderately sized RCTs of risperidone in both adults and children (Reference Shea, Turgay and CarrollShea 2004) have described beneficial effects. Open-label studies with a double-blind discontinuation component have suggested both longer-term benefits and tolerance (e.g. Research Units on Paediatric Psychopharmacology 2005b). In 2006, the US Food and Drug Administration approved risperidone for the symptomatic treatment of irritability (including aggression and self-injury) in children and adolescents. In the UK, a Cochrane review (Reference Jesner, Aref-Adib and CorenJesner 2007) concluded that risperidone can be beneficial, but that the lack of a single standardised outcome measure did not allow direct comparison of studies.
Evidence of efficacy of other atypicals is very preliminary. One small RCT of olanzapine found it to be effective in about 50% of children (Reference Hollander, Wasserman and SwansonHollander 2006a). However, olanzapine is strongly associated with weight gain and other physical morbidity. Open-label trials of quetiapine indicate that the response rate and tolerability are poor (e.g. Reference Martin, Koenig and ScahillMartin 1999). Clozapine and ziprasidone are rarely used because of the risk of blood dyscrasias and QTc prolongation respectively. Open-label trials of aripiprazole in children with autism-spectrum disorders have been encouraging, and seem to be well tolerated (Reference Stigler, Diener and KohnStigler 2009). An RCT in children with autism-spectrum disorders is currently ongoing in the USA (Reference Erickson, Posey and StiglerErickson 2007).
Several large, well-designed RCTs (e.g. Reference Remington, Sloman and KonstantareasRemington 2001) have studied haloperidol and found it efficacious in both children and adults with autism and behaviour problems. Adverse events including dyskinesia and sedation are common, however, and it is therefore more often reserved for treatment-refractory symptoms.
Opiate antagonists
Initial findings of open-label studies of naltrexone seemed promising (Reference Panksepp and LensingPanksepp 1991), but subsequent placebo-controlled studies showed no positive effects for the core social deficits. The most consistent finding is a modest reduction in hyperactivity (Reference Feldman, Kolmen and GonzagaFeldman 1999). A quantitative review of the literature suggests that naltrexone might be beneficial for reducing self-injurious behaviours in individuals with mental retardation, including those with autism-spectrum disorders (Reference Symons, Thompson and RodriguezSymons 2004).
Mood stabilisers
Evidence regarding the effects of anti-epileptic medication is mixed. A small RCT of valproate (Reference Hellings, Weckbaugh and NickelHellings 2005) involving 30 adolescents could not demonstrate a significant difference between the drug and placebo, but a small trial of 13 children treated with divalproex (Reference Hollander, Soorya and WassermanHollander 2006b) demonstrated benefits.
Published data for carbamazepine are limited to case reports. Hepatotoxicity, weight gain, sedation and teratogenicity are important side-effects for this group of drugs. There are no controlled lithium trials in autism-spectrum disorders. Evidence for alpha-2 agonists such as clonidine and beta blockers are limited to small RCTs and case reports (Reference MyersMyers 2007).
Clinical implications
Severe maladaptive aggression and self-injury can be the most disturbing and impairing of co-occurring symptoms in autism, particularly in individuals with intellectual disability. Medication management has a clear role here. Antipsychotics, particularly risperidone, have a consistent evidence base, are increasingly prescribed and can be very useful. However, medication management should be done carefully and well monitored; doses should be titrated up from a low base to the ‘minimum effective dose’ using frequent detailed symptom monitoring. In this way the emergence of medication side-effects (so easily confused with the target symptoms themselves) can be identified and the real effect of the drug can be assessed. Another reason for detailed assessment and targeting of symptoms is that families may be unrealistic about the limitations of medication in complex situations; the placebo response in parental report is often strong. Specific medications should not be pursued if there is no evidence of their benefit; but withdrawal should be slow and judicious to avoid withdrawal effects. The limitations of published studies and drug side-effect profiles should be carefully considered; however, judged carefully and used with persistence, medication management can transform children's development and families' lives.
Hyperkinesis and inattention
Symptoms of hyperkinetic disorder (inattention, impulsivity, distractibility and hyperactivity) are very common in autism, particularly in children and adolescents (Reference Lee and OusleyLee 2006). Traditionally in UK diagnostic schemes, a diagnosis of hyperkinetic disorder is not made if it occurs exclusively during the course of an autism-spectrum disorder (the diagnostic hierarchy concept). However, this has the limitation of tending to underplay or even obscure significant and treatable symptom co-occurrence. These symptoms may severely impair an individual's functioning and warrant treatment in their own right. ICD–11 is likely to relax this strictly hierarchical approach to diagnostic formulation.
Psychostimulants
Randomised controlled trials of methylphenidate have demonstrated improvement in childhood autism (Reference Quintana, Birmaher and StedgeQuintana 1995; Research Units on Paediatric Psychopharmacology Autism Network 2005a). This applies to immediate-release preparations but it is unclear whether the results can be applied to other stimulants. Trials suggest that the response rate in people with autism is lower than that of people without autism, and that side-effects such as irritability and poor appetite are more common (Reference Erickson, Posey and StiglerErickson 2007). One trial suggested that patients with Asperger syndrome may respond more positively than those with other autism-spectrum disorders, but not better than neurotypical individuals (Reference Stigler, Desmond and PoseyStigler 2004). However, a more recent, large open-label study found no statistically significant difference in degree of response or adverse events between those with autism-spectrum disorders and those without (Reference Santosh, Baird and PityaratstianSantosh 2006). Secondary analysis of the Research Units on Paediatric Psychopharmacology Autism Network (2005a) trial also suggested that psychostimulants may have a positive effect on some aspects of social communication in children with autism-spectrum disorders and hyperactivity, such as joint attention initiations (Reference Jahromi, Kasari and McCrackenJahromi 2009).
Atomoxetine
Atomoxetine is a selective noradrenaline inhibitor. Recently, open-label studies and a small pilot RCT (e.g. Reference Arnold, Aman and CookArnold 2006) have suggested effectiveness for hyperactivity, impulsivity and oppositional behaviour in autism-spectrum disorders. Common side-effects include nausea, increased heart rate and fatigue.
Alpha-2 adrenergic agonists
Two very small RCTs involving a total of 17 patients (Reference Fankhauser, Karumanchi and GermanFankhauser 1992) have suggested modest benefits of clonidine, and one small crossover study suggests similar effects for lofexidine (Niederhofer 2002). One open-label trial indicates that guanfacine may also be helpful for hyperactivity (Reference Scahill, Aman and McDougleScahill 2006). Common side-effects include sedation and hypotension.
Cholinergics
Open-label trials and case series of the acetylcholinesterase inhibitors donepezil, galantamine and rivastigmine have reported improvements in hyperactivity, inattention and irritability (Reference Chez, Aimonovitch and BuchananChez 2004).
Amantadine
One RCT of amantadine for children (Reference Ing, Wright and HandenIng 2001) showed some improvements in hyperactivity and impulsivity on observer measures but not on parent-scored measures. Amantadine may therefore be useful in modulating behaviour in some young patients, but it is not clear whether this is because of its glutamatergic activity or enhancement of dopaminergic neuro-transmission.
Clinical implications
In children with any form of developmental disability, hyperactivity and inattention are more complex to treat than in neurotypical children: response is more idiosyncratic and unwanted effects are more common. Thus far, immediate-release methylphenidate has the most convincing evidence base with regard to symptoms of motor hyperactivity and inattention in children and adolescents. Research involving adults with autism-spectrum disorders who experience these symptoms, as in the non-autism adult population with such problems, continues to lag far behind. Recent National Institute for Health and Clinical Excellence guidelines (National Collaborating Centre for Mental Health 2008) do not specifically mention individuals with autism-spectrum disorders who also have significant problems with hyperkinesis. However, they state that drug treatments should be offered as first-line treatment only to patients with severe symptoms or impairment, and always in combination with psychosocial interventions. Methylphenidate, dexamfetamine and atomoxetine are all regarded as options for management.
Mood disorders
Patients with autism have been reported to be at increased risk of depression (prevalence of about 2% of all autism-spectrum disorders), particularly those who are more cognitively able (Reference Ghaziuddin, Ghaziuddin and GredenGhaziuddin 2002). However, a recent follow-up study suggests that the incidence of mood disorder may be no different from the general population (Reference Hutton, Goode and MurphyHutton 2008). Nevertheless, depressive symptoms remain an important treatable cause of deterioration in functioning.
Mood disorder tends to emerge from middle childhood and is often associated clinically with the child's increased self-awareness of difference and the increasing social demands of peers. As in other childhood mood disorders, psychosocial management is the first-line approach, and adaptation of the social and educational environment is often a first-line target.
For persistent and/or severe depressive disorder in children and adolescents, open-label trials of SSRIs, including fluoxetine, fluvoxamine, sertraline, citalopram and escitalopram, in children and adults have been associated with improvements in a depressive phenotype, including such symptoms as social withdrawal, irritability, sadness or crying, decreased energy and weight loss. Systematic reviews have confirmed that SSRIs should be considered for the treatment of depressive symptoms in autism (Reference Posey, Erickson and StiglerPosey 2006).
Anticonvulsants such as divalproex have been posited to be useful in mood lability (Reference MyersMyers 2007). Several case reports describe patients with autism and atypical bipolar disorder who responded well to open-label treatment with lithium (e.g. Reference Kerbeshian, Burd and FisherKerbeshian 1987).
Anxiety disorders
Individuals with autism-spectrum disorders show increased risk of anxiety disorders (Reference Kim, Szatmari and BrysonKim 2000). It is often particularly intense and sometimes atypical; sometimes interacting with core symptomatology to even mimic thought disorder. One double-blind trial shows evidence of efficacy using fluvoxamine (Reference McDougle, Naylor and CohenMcDougle 1996), and case reports of other SSRIs indicate improvements in anxiety symptoms with their use (Reference Posey, Erickson and StiglerPosey 2006).
An open-label study as well as case reports suggest that buspirone (a 5-HT agonist) may also be effective for anxiety (Reference Buitelaar, van der Gaag and van der HoevenBuitelaar 1998a). Beta blockers may be appropriate where panic is a component symptom.
Clinical implications
Anxiety in autism-spectrum disorders has particular qualities and is often underrecognised. It can significantly increase presenting social symptomatology and social anxiety, or specific phobias can contribute to social avoidance and functional social impairment. Anxiety management strategies and desensitisation for behavioural avoidance can usefully be combined with medication management.
Tic disorders and OCD
Tourette syndrome has been shown to be comorbid in over 6% of autism cases, more than would be expected by chance (Reference Baron-Cohen, Mortimore and MoriartyBaron-Cohen 1999). There are increasingly recognised overlaps between autism-spectrum disorders, OCD and tic disorders. There is no evidence that pharmacological treatment of these disorders should differ from that used in each disorder alone. The use of haloperidol, clonidine and risperidone for tics, and clomipramine or SSRIs for obsessions and compulsions, is well described in the literature (Reference GringrasGringras 2000).
Sleep disorders
There is some evidence of abnormal melatonin regulation in autism (Reference Paavonen, Nieminen-von Wendt and VanhalaPaavonen 2003). Open-label studies suggest melatonin may be effective for improving sleep onset, but controlled trials are lacking (Reference GringrasGringras 2000). Alpha-2 agonists, antihistamines, ramelteon (a melatonin receptor agonist) and mirtazapine have all been reported to have some effect on sleep in open-label trials and case reports (e.g. Reference Owens, Babcock and BlumerOwens 2005).
Clinical implications
Sleep disorder is common and often under-recognised. It is the cause of long-term debility for families and daytime symptoms in patients. Many parents are focused on medication management for these problems, and they can be effective –equally important is systematic sleep hygiene and behavioural measures.
Thought disorder
There is consensus in clinical literature on the occurrence of atypical quasi-psychotic symptomatology in autism. This is often transient but can be recurring or persistent in mild form. Such symptomatology is important to recognise as it often causes diagnostic confusion and inappropriate treatment response. The nosological status of such states is not agreed: they do not necessarily represent a psychotic prodrome and the prevalence of psychosis is not markedly increased. The acute transient form can often be appropriately classified as a brief reactive psychosis; a useful syndromic account of the relapsing form has been given under the descriptive if inelegant term of ‘multiplex complex developmental disorder’ (Reference Buitelaar and van der GaagBuitelaar 1998b).
These states can often be managed conservatively without medication by identification and reduction of relevant environmental stressors. However, the more recurrent forms will often need medication management, and the correct response here is for titrated antipsychotic medication, often with low-dose maintenance treatment to prevent a relapsing course. There are rarer states that also need consideration in a differential diagnosis, such as emerging developmental abnormalities in adolescence associated with microdeletion on 22q11 (the velocardiofacial syndrome).
Discussion
Research in many of the areas discussed is still in its early phases, and the ongoing limitations of the evidence and intrinsic difficulties of measurement are important for clinicians to remember when discussing things with often well-informed and frustrated families. Box 1 highlights some of the evidential shortcomings of published literature in this field. There are few RCTs, and trials are often small and underpowered. Long-term studies of unwanted effects are often lacking. The population studied is not homogeneous and many trials use disparate outcome measures, which makes comparisons difficult or simply invalid. Many outcome measures subsume a variety of elements of behaviour, making it hard to be clear about what the data actually show for specific areas (Reference Wisniewski, Brimacombe and MingWisniewski 2007).
BOX 1 Shortcomings of published data
-
• Few randomised controlled trials
-
• Underpowered trials
-
• Short follow-up periods
-
• Lack of safety data
-
• Lack of trials in adult populations
-
• Heterogeneous populations studied (need for appropriate case definitions)
-
• Heterogeneous clinical outcome measures used make it difficult to compare trials
-
• Power of placebo response
-
• Problems in measuring change in people with autism
Moreover, there is the inherent difficulty of studying pharmacological response in developmental disorders themselves. Measuring change in autism-spectrum disorders is complex and must take into account day-to-day fluctuations, powerful placebo effects and idiosyncratic responses. The examples of fenfluramine and secretin illustrate these particular difficulties. Reasons for a large placebo response in children with autism include heightening of positive expectancy by media attention, by sensory experiences associated with intravenous injections, and participation effects modifying caregivers' behaviour. Reference SandlerSandler (2005) discusses these issues in detail.
The underlying developmental trajectory of individual patients is highly variable and may confound apparent positive responses to medication. This issue is more widely recognised now and researchers (e.g. Research Units on Paediatric Psychopharmacology Autism Network 2005a,b; Reference Scahill, Aman and McDougleScahill 2006) are attempting to address these concerns; however, the field awaits a critical mass of larger, more robust clinical trials with more specific measurements and sophisticated analyses of clinical effectiveness. Clinicians will need to carefully interpret the clinical evidence and make active adjustments in applying it to the specific situation of their patients.
Despite the limitations, however, both the quantity and quality of medication trials targeting symptom domains in autism-spectrum disorders have increased in recent years. There remains a lack of important information on long-term safety and efficacy of drugs, and the standard of evidence so far does not allow for definitive treatment protocols for various symptom clusters. However, the aggregation of data suggests that SSRIs, risperidone and immediate-release methylphenidate can be of great value within the domains discussed. There are as yet no proven treatments for the underlying social deficit in autism.
We have emphasised that medication management is only one strand of interventions for people with autism and the mainstay of treatment remains educational and psychosocial. Pharmacological management needs to be undertaken by thorough assessment, accurate diagnosis and regular monitoring of target symptom clusters, comorbid diagnoses and response to treatment (Box 2).
BOX 2 Important considerations when prescribing for autism
To make informed decisions about a potential role for medication, the prescriber must:
-
• clarify characteristics of the challenging behaviours including frequency, intensity, duration and degree of interference with functioning
-
• be clear about the target symptomatology to be treated (differentiate core from comorbid symptoms)
-
• identify exacerbating and ameliorating factors, including response to psychotherapeutic interventions
-
• assess existing and available health, educational and social supports, and the strengths of the family (e.g. the family's ability to support the individual)
-
• assess comorbid physical problems by thorough history and examination, and consider their impact on presentation and treatment of the challenging behaviours
-
• consider potential adverse events and drug interactions
-
• avoid polypharmacy
-
• use a ‘start low and go slow’ treatment strategy.
Looking to the future
There are several exciting new developments within the field of pharmacology research in autism-spectrum disorders. Large trials in glutamatergic function are currently ongoing, particularly in the USA. Most studies to date focus on the use of one drug to target one group of related symptoms. One such study is an ongoing RCT of aripiprazole v. placebo in the reduction of aggressive and aberrant behaviour in children with autism (principal investigator: S. L. Novotny; http://clinicaltrials.gov/ct2/show/NCT00468130). Studies using more than one drug to target more than one symptom domain (so called ‘coactive studies’) are also being considered in treatment trials: d-cycloserine and aripiprazole are currently the subjects of a large trial to target both social impairment and the domain of aggression/self-injury (Reference McDougle, Stigler and EricksonMcDougle 2006). Researchers are beginning to consider the value of a more formal combination of behavioural and medical interventions in complex treatment trials designed to alter the developmental trajectory of those with autism. Advances in neurophysiology and genetics may also make it possible to delineate subgroups that may be particularly responsive to particular treatments. Such developments may pave the way for a more integrated consensus on an overall approach to treatment of autism-spectrum disorders across the lifespan.
MCQs
Select the single best option for each question stem
-
1 Selective serotonin reuptake inhibitors:
-
a are of proven efficacy in the treatment of core social impairment in autism
-
b may cause behavioural activation in children with autism
-
c are not indicated for the treatment of obsessions and compulsions in autism-spectrum disorders
-
d have been demonstrated to be safe in the long-term treatment of patients with autism
-
e are not indicated for the treatment of interfering ritualised behaviours in adolescents with autism.
-
-
2 Which of the following is considered an effective treatment for anxiety symptoms in autism-spectrum disorders?
-
a methylphenidate
-
b risperidone
-
c buspirone
-
d d-cycloserine
-
e vitamin B6.
-
-
3 Which of the following is considered an effective treatment for aggressive and/or self-injurious behaviour in autism?
-
a quetiapine
-
b clomipramine
-
c methylphenidate
-
d atomoxetine.
-
e risperidone.
-
-
4 Risperidone:
-
a may lead to clinically significant increases in prolactin levels in patients with autism
-
b is of proven safety in the treatment of behaviours associated with autism in the elderly population
-
c is never indicated in the treatment of aggression and severe self-harm in patients with autism
-
d is considered an effective treatment for depressive symptoms
-
e always leads to significant QTc prolongation in ECGs of children with autism.
-
-
5 Research evidence shows that:
-
a the ratio of male:female patients with autism-spectrum disorders is 8:1
-
b the prevalence of narrowly defined autism is 2 per 1000 children
-
c up to 75% of people with autism are prescribed at least one psychotropic medication in the UK
-
d children with autism and hyperkinetic symptoms do not respond to immediate-release methylphenidate preparations
-
e diagnostic criteria for psychiatric disorders do not have to be modified in patients with autism and intellectual disability.
-
MCQ answers
| 1 | b | 2 | c | 3 | e | 4 | a | 5 | c |
TABLE 1 SELECTED medications for use in domains of behaviour in autism
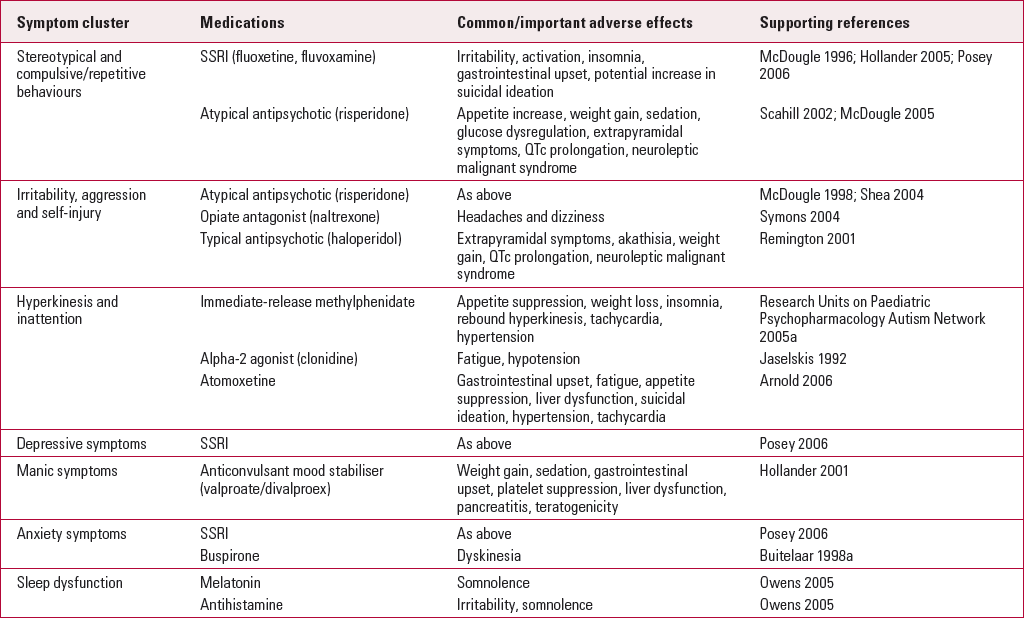
| Symptom cluster | Medications | Common/important adverse effects | Supporting references |
|---|---|---|---|
| Stereotypical and compulsive/repetitive behaviours | SSRI (fluoxetine, fluvoxamine) | Irritability, activation, insomnia, gastrointestinal upset, potential increase in suicidal ideation | Reference McDougle, Naylor and CohenMcDougle 1996; Reference Hollander, Phillips and ChaplinHollander 2005; Reference Posey, Erickson and StiglerPosey 2006 |
| Atypical antipsychotic (risperidone) | Appetite increase, weight gain, sedation, glucose dysregulation, extrapyramidal symptoms, QTc prolongation, neuroleptic malignant syndrome | Reference Scahill, McCracken and McGoughScahill 2002; Reference McDougle, Scahill and AmanMcDougle 2005 | |
| Irritability, aggression and self-injury | Atypical antipsychotic (risperidone) | As above | Reference McDougle, Holmes and CarlsonMcDougle 1998; Reference Shea, Turgay and CarrollShea 2004 |
| Opiate antagonist (naltrexone) | Headaches and dizziness | Reference Symons, Thompson and RodriguezSymons 2004 | |
| Typical antipsychotic (haloperidol) | Extrapyramidal symptoms, akathisia, weight gain, QTc prolongation, neuroleptic malignant syndrome | Reference Remington, Sloman and KonstantareasRemington 2001 | |
| Hyperkinesis and inattention | Immediate-release methylphenidate | Appetite suppression, weight loss, insomnia, rebound hyperkinesis, tachycardia, hypertension | Research Units on Paediatric Psychopharmacology Autism Network 2005a |
| Alpha-2 agonist (clonidine) | Fatigue, hypotension | Reference Jaselskis, Cook and FletcherJaselskis 1992 | |
| Atomoxetine | Gastrointestinal upset, fatigue, appetite suppression, liver dysfunction, suicidal ideation, hypertension, tachycardia | Reference Arnold, Aman and CookArnold 2006 | |
| Depressive symptoms | SSRI | As above | Reference Posey, Erickson and StiglerPosey 2006 |
| Manic symptoms | Anticonvulsant mood stabiliser (valproate/divalproex) | Weight gain, sedation, gastrointestinal upset, platelet suppression, liver dysfunction, pancreatitis, teratogenicity | Reference Hollander, Dolgoff Caspar and CartwrightHollander 2001 |
| Anxiety symptoms | SSRI | As above | Reference Posey, Erickson and StiglerPosey 2006 |
| Buspirone | Dyskinesia | Reference Buitelaar, van der Gaag and van der HoevenBuitelaar 1998a | |
| Sleep dysfunction | Melatonin | Somnolence | Reference Owens, Babcock and BlumerOwens 2005 |
| Antihistamine | Irritability, somnolence | Reference Owens, Babcock and BlumerOwens 2005 |




eLetters
No eLetters have been published for this article.