The disciplines of psychiatry and neurology have shared a chequered past. At times united, then divided, their current realignment in neuropsychiatry seeks to combine not only the neurological and psychiatric perspectives of brain disorders but also each sub-specialty’s skills in their assessment and management. In this review, we discuss how recent advances have informed the neuropsychiatric management of various neurological illnesses. (We have not considered the dementia syndromes or presenile dementias.) Good-quality evidence regarding therapeutic approaches, whether biological or psychological, is often lacking. However, where possible we have highlighted appropriate therapies and treatment strategies.
Focal neurological disorders
Stroke and traumatic brain injury
Both stroke and traumatic brain injury are characterised by acute, usually focal, brain injuries that result in neurological disability, with a subsequent recovery period. The neuropsychiatric presentations associated with these injuries are similar and therefore considered together. Following both stroke and traumatic brain injury, neurological symptoms (such as hemiparesis or dysphasia) are the main cause of disability, but neuropsychiatric symptoms are likely to have a greater negative impact on disability and, possibly, mortality.
Prevalence of neuropsychiatric disorders
Table 1 illustrates the range and prevalence of neuropsychiatric sequelae observed after stroke. Very similar problems, by and large with similar prevalence rates, are observed after traumatic brain injury. However, traumatic brain injury is associated with higher rates of bipolar affective disorder, and particularly of rapid-cycling bipolar disorder with a periodicity of days rather than weeks.
Clinical presentations
Mood disorders
Mood disorders are common in both stroke and traumatic brain injury. They are generally similar to the disorders experienced by people without brain injury, although there is often more evidence of lability of mood and apathy.
Stroke must be regarded as a significant risk factor for suicide (Reference Stenager, Madsen and StenagerStenager et al, 1998). After traumatic brain injury, the standardised mortality rate from suicide is increased threefold, so that about 1% will die by suicide over a 15-year follow-up, with the risk remaining fairly constant over this period (Reference Teasdale and EngbergTeasdale & Engberg, 2001). A study of suicidality after traumatic brain injury found that 35% of individuals had clinically significant levels of hopelessness, 23% had suicidal ideation and 17% had attempted suicide during the 5 years since their injury (Reference Simpson and TateSimpson & Tate, 2005).
Psychosis
Psychosis is a rare but serious complication after traumatic brain injury, presenting both early and late in recovery. When early, it is more likely to be associated with delirium. Head injury has long been thought a possible risk factor for schizophrenia, although a comprehensive critical review of psychosis following head injury concludes that the injury is unlikely to be causal in the disorder (Reference David and PrinceDavid & Prince, 2005).
Progressive cognitive decline
Brain injury may also be followed by progressive cognitive decline. Dementia pugilistica may develop years after repeated blows to the head, usually in boxers. Head injuries, particularly in men, may predispose to the development of Alzheimer’s disease (Reference Fleminger, Oliver and LovestoneFleminger et al, 2003b ). This is presumably due to deposition of β-amyloid in the brain at the time of injury, although evidence that it is linked to APOE status is inconsistent.
Other neuropsychiatric presentations
Agitation and aggression are relatively infrequent after stroke but are often seen after traumatic brain injury. This partly reflects the individual’s personality. Agitation is common in association with the post-traumatic confusional state (see Delirium below). Early agitation predicts long-term, typically explosive aggressive behaviour.
Thoughtlessness, impulsiveness and irritability are particularly troublesome changes in personality after traumatic brain injury, and a common cause of distress for family and carers. Apathy and poor motivation often compound the problems. Personality change is frequently associated with dysexecutive syndrome, which is manifested in impaired problem-solving owing to difficulties in planning, prioritising and monitoring tasks, and in multi-tasking. Impairments of concentration and memory and psychomotor slowing are also common cognitive symptoms.
Associated conditions
In post-concussion syndrome it can be difficult to disentangle the relative contributions of brain injury and psychological responses. Symptoms include double and blurred vision, noise sensitivity, dizziness, difficulties concentrating, fatigue and head and neck pains. Anxiety and depression tend to occur later. Similar non-specific symptoms are seen in somatisation disorders, including chronic fatigue syndrome. Involvement in a compensation claim may increase post-concussion symptoms, particularly after mild injury such as whiplash.
The psychological symptoms after whiplash are similar to those of post-concussion syndrome. A protracted course, beyond 6 months, is described as late whiplash syndrome. A controlled study of psychological outcomes and predictors following whiplash, compared with other road accident injury, showed no injury-specific psychiatric factors between groups (Reference Mayou and BryantMayou & Bryant, 2002). Of particular note was the association between claiming compensation at 3 months and pain at 1 year.
Aetiology
There has been a long-standing debate as to whether there is an association between the location of the brain lesion (particularly anterior, left-hemisphere lesions) and post-stroke depression. A systematic review suggested that there is no evidence to support such a relationship (Reference Carson, MacHale and AllenCarson et al, 2000). However, the methodology of this review, particularly the exclusion of some studies, has been criticised. A subsequent meta-analysis re-ignited the debate and suggested that proximity of the lesion to the left frontal pole predicts depressive illness (Reference Narushima, Kosier and RobinsonNarushima et al, 2003). This was further supported by another appraisal from Finland (Reference Vataja, Leppavuori and PohjasvaaraVataja et al, 2004).
Most traumatic brain injury is caused by closed head injury and is often complicated by alcohol. The vulnerability to contusions of areas of the brain that are involved in social behaviour, cognition and regulation of mood (medial orbitofrontal and anterior temporal lobes) (Reference Tranel, Bechara, Damasio and GazzanigaTranel et al, 2000) partly explains why the neuropsychiatric consequences of traumatic brain injury usually outstrip the neurological sequelae as predictors of outcome. The best predictor of outcome is the duration of post-traumatic amnesia, the period from injury to the return of continuous day-to-day memory. People with post-traumatic amnesia that lasts longer than 1 month are unlikely to return to work.
Treatment
It is important to be vigilant in identifying post-stroke depression, and treatment of depressive symptoms should always be considered (Box 1). The fact that they are ‘understandable’ or are symptoms that may be a direct consequence of brain injury (e.g. apathy) should not prevent a trial of an antidepressant. It has been suggested, on the basis of limited evidence, that the D2-receptor agonist bromocriptine might have therapeutic benefit in the management of apathy.
Box 1 National clinical guidelines for the management of mood disturbance after stroke
-
• Provide information, advice and the opportunity to talk about the impact of the stroke
-
• Assess psychosocial needs
-
• Screen for depression and anxiety within the first month of stroke and monitor mood
-
• With those who can respond use standardised questionnaires for screening
-
• Confirm emotionalism by a few simple questions at interview
-
• If one mood disorder is present assess for the others
-
• Severe, persistent or troublesome tearfulness (emotionalism) should be treated with anti-depressants; the frequency of crying should be monitored to check effectiveness
-
• Consider a trial of antidepressant medication in those with persistently depressed mood of at least 1 month’s duration
-
• If there is a good response, antidepressants should be continued for at least 6 months
After Intercollegiate Working Party for Stroke (2004: pp. 53–54).
There is very limited evidence from systematic reviews regarding the efficacy of antidepressants in treating post-stroke depression (Reference Turner-Stokes and HassanTurner-Stokes & Hassan, 2002). Although there is the suggestion that tricyclic antidepressant drugs are more effective, selective serotonin reuptake inhibitors (SSRIs), having fewer side-effects, are a reasonable first choice (Box 2).
Box 2 Factors influencing choice of psychotropic medications
-
• Side-effects of the medication, particularly on cognitive function as a result of anticholinergic effects
-
• Whether the drug is generally sedative, useful in agitation or anxiety, or alerting
-
• Drug interactions, particularly with anticoagulant, cardiac and antiparkinsonian medications
-
• Drug efficacy in the population of interest
-
• Potential for reducing seizure threshold
There is increasing interest in the early prescription of antidepressants after stroke as a preventive strategy for mood disorder. However, a review of treatment trials found no prophylactic effect of antidepressants compared with placebo (Reference Anderson, Hackett and HouseAnderson et al, 2004). It has been suggested that treatment of post-stroke depression in the first month when compared with the third month benefits functional outcome at up to 2 years (Reference Narushima and RobinsonNarushima & Robinson, 2003).
If all else fails, electroconvulsive therapy should be considered. This has been shown to be safe for the treatment of depression after both stroke and traumatic brain injury (Reference Currier, Murray and WelchCurrier et al, 1992).
Antidepressants are useful in treating emotionalism (lability of mood, emotional incontinence), regardless of the presence of depression.
Several psychotropic drugs have been used in the management of agitation and/or aggression in people with acquired brain injury, without clear evidence of their efficacy. A review (Reference Fleminger, Oliver and GreenwoodFleminger et al, 2003a ) reported that beta-blockers have the best evidence supporting their efficacy in treating these behavioural symptoms. None the less, in view of the large doses used in these studies, which were associated with significant side-effects, they should be prescribed with caution and, ideally, with the patient’s consent. Carbamazepine is commonly used as a first-choice medication in this setting, despite the lack of research evidence for its efficacy. Sodium valproate may be used as an alternative. Response to medication is usually seen early, within the first 6 weeks, and it is suggested that this is an adequate period during which clinical benefit should be expected before switching to an alternative treatment. There is no evidence that aggression is different from agitation in terms of its response to medication (Reference FlemingerFleminger, 2003).
Olanzapine or quetiapine are reasonable antipsychotics for psychosis associated with traumatic brain injury, because they have fewer extrapyramidal side-effects.
There is little evidence for psychological interventions for post-stroke depression. Of those used in case studies and series reviewed by Reference Kneebone and DunmoreKneebone & Dunmore (2000), cognitive–behavioural therapy (CBT) showed the most promise. However, a small randomised controlled trial reported subsequent to this found CBT to be ineffective (Reference Lincoln and FlannaghanLincoln & Flannaghan, 2003). Similarly, for other acquired brain injuries, there is only scarce evidence supporting such psychological strategies (Reference Khan-Bourne and BrownKhan-Bourne & Brown, 2003). Early educational interventions after traumatic brain injury may prevent later symptoms.
Multiple sclerosis
Prevalence of neuropsychiatric disorders
The neuropsychiatric presentations of this multi-focal demyelinating disorder are varied and include mood, anxiety, cognitive and psychotic disorders. It has been suggested by several studies that the prevalence of depression in multiple sclerosis is higher than in control groups with different neurological illnesses. However, methodological problems have been cited, particularly with regard to clinician masking and diagnostic criteria. A lifetime prevalence for depressive symptoms of 40–50% is generally accepted (Reference Siegert and AbernethySiegert & Abernethy, 2005). Psychotic illness in multiple sclerosis is most commonly observed in the context of treatment with steroids, where it is most often an affective psychosis, although schizophreniform psychoses are also seen.
Cognitive impairment in multiple sclerosis has been estimated to have a prevalence of up to 50% in community samples.
Clinical presentations
Some depressive symptoms are probably more common than others in multiple sclerosis. Of these, fatigue and cognitive impairment have received the most attention. Generally, older studies have suggested that neither of these is correlated with depression in the disorder. However, more recent studies have supported positive correlations, suggesting that mental fatigue (Reference Schreurs, de Ridder and BensingSchreurs et al, 2002) and impaired effortful information processing (Reference Arnett, Rao and GrafmanArnett et al, 1997) in particular are features of the depression. Suicide rates in people with multiple sclerosis have been shown to be twice the mean rate seen in the adjusted population (Reference Stenager, Stenager and Koch-HenricksenStenager et al, 1992) and are associated with living alone, alcohol misuse and depressive disorder.
Cognitive deficits arise early in the course of multiple sclerosis and often before the diagnosis has been made. The course of cognitive decline appears to be slow in the majority of cases, although risk factors for a more rapid decline include disease progression, age and worsening of physical disability. Particular impairment is seen in verbal fluency, comprehension, naming and executive dysfunction, as well as memory.
Aetiology
Beta-interferon has been implicated as a cause of depression, although the evidence for this is contentious. Neuroimaging studies of lesion location have been conducted in individuals with multiple sclerosis and depression or psychosis. The most robust findings in depression implicate more hyperintense lesions in left inferior medial frontal regions and greater atrophy of left anterior temporal regions (Reference Feinstein, Roy and LobaughFeinstein et al, 2004). It has been suggested that location of temporal lobe lesion may correlate with psychotic illness (Reference Feinstein, du Boulay and RonFeinstein et al, 1992).
Treatment
Desipramine has been shown to be more effective than placebo for depression in multiple sclerosis, although anticholinergic side-effects may preclude its use. The SSRIs are a good first-line choice although they can cause sexual dysfunction.
Limited evidence exists regarding the best choice of antipsychotic for treatment of psychosis in multiple sclerosis, although an atypical antipsychotic is a sensible choice.
People with multiple sclerosis treated in an open trial of donepezil showed a reduction in cognitive impairment (Reference Greene, Tariot and WishartGreene et al, 2000).
Individuals with fatigue may benefit from approaches used for chronic fatigue syndrome. Cognitive–behavioural therapy for depression is likely to be useful and it has been found to have similar outcomes to treatment with an SSRI (Reference Mohr, Boudewyn and GoodkinMohr et al, 2001).
Epilepsy and non-epileptic seizures
Prevalence of neuropsychiatric disorders
Neuropsychiatric disorder has been estimated to occur in 6% of people with epilepsy, rising to 10–20% in those with temporal lobe and/or refractory epilepsy. Non-epileptic seizures, which come under the rubric of conversion disorders or dissociative states, are an important differential diagnosis of epilepsy. It has been estimated that between 9 and 50% of patients referred to specialist epilepsy centres have non-epileptic episodes (Reference Francis and BakerFrancis & Baker, 1999). The most common neuropsychiatric disorders associated with epilepsy and their prevalence are summarised in Table 2.
Clinical presentations
In all presentations in epilepsy, it is important to distinguish between symptoms that are associated with seizures and those that are independent of seizures. Perhaps most importantly, differentiation of the relationship of neuropsychiatric presentations to the seizure, i.e. whether they are pre-ictal, post-ictal or inter-ictal, is paramount in guiding treatment planning, as this may influence whether the clinician manipulates anti-epileptics, or adds psychiatric medications or does both (see below).
Mood disorders
As with other neuropsychiatric disorders in this setting, depression can be related to pre-ictal events or can be inter-ictal. There has been recent interest in inter-ictal dysphoric disorder, in which people with temporal lobe epilepsy experience at least three symptoms of depressed mood, pain, irritability, decreased energy, sleeplessness, anxiety and intense fear or euphoria (Reference Blumer, Montouris and DaviesBlumer et al, 2004).
Psychoses
Post-ictal psychosis often follows an increase in seizure frequency or a cluster of seizures. It commonly presents with changes in mood, paranoid delusions and hallucinations. These often remit spontaneously within days.
Inter-ictal psychosis resembles schizophrenia, but presents more frequently with visual rather than auditory hallucinations.
Non-epileptic seizures
There is no completely reliable method of discerning dissociative from epileptic seizures. Dissociative seizures can co-exist with epilepsy, and are associated with sexual abuse and female gender. Assessment should include thorough history-taking (Box 3), an electroencephalogram (EEG) and careful description or, preferably, video recording of pre-ictal, ictal and post-ictal events. Increased post-ictal serum prolactin concentrations (>1000 IU/l) despite normal baseline levels are found after epileptic seizures (Reference Brown and TrimbleBrown & Trimble, 2000), but measurements must be taken within 15 min of the event.
Box 3 Factors in the history that may support a diagnosis of non-epileptic seizures
-
• Past psychiatric history
-
• More likely to express distress in a physical way
-
• History of social stressors
-
• Seizures more likely to happen in the daytime and when others are present
-
• Less likely to sustain injury from seizures
-
• More likely to maintain body tone during a seizure
-
• Regaining alertness and orientation rapidly
-
• Ability to recall events clearly
Aetiology
Combinations of biological and psychosocial factors are likely to play a role in the aetiology of neuropsychiatric disorders in epilepsy. In depressive disorders, these factors include anti-epileptic-induced effects such as folate deficiency and frontal lobe dysfunction. In psychoses, hippocampal damage and chronic inhibitory neurophysiological changes may be involved. The hypothesis of forced normalisation, where the abnormal EEG is normalised by anti-epileptic drugs, remains popular. This is based on the observation that the EEG recordings of patients normalised during psychotic episodes and that normalisation induced by anti-epileptics may be an aetiological factor for psychoses. Forced normalisation has been reported in both temporal lobe and generalised epilepsies. A paranoid psychosis has been its most frequent manifestation and it has been observed following the use of various medications, including phenytoin, carbamazepine, ethosuximide and levetiracetam. Forced normalisation may not be restricted to psychoses in epilepsy, but may also present as episodes of major depression.
The social stigma associated with epilepsy is a key psychosocial risk. A critical review of studies of the aetiology of non-epileptic seizures reported that a history of abuse or trauma was indentified in etween 15 and 40% more people who experienced seizures than in control groups (Reference Fiszman, Alves-Leon and NunesFiszman et al, 2004).
Treatment
It is important to identify the relationship of the psychiatric disturbance to ictal events, the role of anti-epileptic drugs in the aetiology of symptoms and the importance of psychosocial factors. For symptoms related directly to the seizure itself, optimal control of seizures is the treatment of choice.
When considering psychotropic medication for people with epilepsy there is always the concern that the medication will decrease the seizure threshold. However, a retrospective study has suggested that psychotropic medication does not increase mean seizure frequency (Reference Gross, Devinsky and WestbrookGross et al, 2000). It is important to start psychotropic medication at a low dose and increase it slowly. The SSRIs may be less likely to reduce the seizure threshold than tricyclic antidepressants. Because there may be an interaction between psychotropics and anticonvulsants (for example fluoxetine may increase carbamazepine levels), levels of anticonvulsant should be closely monitored.
It should also be borne in mind that anti-epileptic drugs, often also used routinely to stabilise mood, can be associated with adverse neuropsychiatric reactions. Valproate-induced hyperammonaemic encephalopathy, which presents with impaired consciousness and focal neurological signs, may well go unrecognised if somnolence, decreased motor activity and increased lethargy are misinterpreted as a therapeutic response in patients with mania. Additional symptoms may include subtle personality change, confusion, vomiting and hyperventilation. These clinical features of hyperammonaemia are very variable, can be episodic and are often difficult to detect early. Being aware of a history of similar symptoms and a family history is useful. Serum ammonia levels should be measured and expert advice sought (Reference Hawkes, Thomas and JurewiczHawkes et al, 2001).
Haloperidol and sulpiride may be less epileptogenic than other antipsychotics, and therefore the treatment of choice for epileptic psychoses. There is limited clinical experience and few systematic studies of atypical antipsychotics in epilepsy and they should be used with care. Clozapine is particularly epileptogenic and, as always, a balance of risks and benefits must be considered, discussed with the patient and carefully documented when commencing atypical antipsychotic treatment in epilepsy.
Recent work suggests that CBT may be effective in the treatment of non-epileptic seizures (Reference Goldstein, Deale and Mitchell-O'MalleyGoldstein et al, 2004).
Disorders of the basal ganglia
Several neural circuits that link the frontal cortex, thalamus and basal ganglia are involved in movement, attention, memory and reward processes. This may explain why disorders of the basal ganglia are characterised by abnormalities of movement, mental state and cognitive function (Reference Ring and Serra-MestresRing & Serra-Mestres, 2002). In this section we briefly discuss Parkinson’s and Huntington’s diseases.
Parkinson’s disease
Prevalence of neuropsychiatric disorders
The common neuropsychiatric presentations and their prevalence rates are outlined in Table 3.
Clinical presentations
Mood disorders
Depression is associated with faster disease progression and more rapid decline in cognitive function and activities of daily living, and is a risk factor for dementia in Parkinson’s disease.
Other neuropsychiatric presentations
Psychosis in Parkinson’s disease, often with visual hallucinations, persecutory delusions and pathological jealousy, is associated with cognitive impairment and recent increases in antiparkinsonian medication.
The differences between the dementia of Parkinson’s disease and Lewy body dementia remain a topic of debate. Features of Lewy body dementia include fluctuating cognition, visual hallucinations and parkinsonism. The diagnosis cannot be made if parkinsonism developed more than 1 year before the onset of dementia.
In hedonistic homoeostatic dysregulation people with Parkinson’s disease take increasingly more dopamine-replacement medication, particularly subcutaneous apomorphine, despite dyskinetic adverse effects. They also show hypersexuality, hypomanic symptoms and pathological gambling and shopping while on high doses of dopamine agonists. This uncommon neuropsychiatric syndrome causes significant distress to patients and carers and its recognition is paramount in guiding appropriate management, as it can be commonly mistaken for other neuropsychiatric presentations.
Aetiology
It is probable that both biological and psychological factors explain depression in Parkinson’s disease:
-
• biological – loss of monoaminergic neurons; hypometabolism in caudate, inferior orbitofrontal and medial frontal regions on positron emission tomography
-
• psychological – chronic disabling illness, psychosocial stress.
As previously stated, psychoses in Parkinson’s disease are often related to recent increases in antiparkinsonian medications. History of psychiatric illness, personality disorder or a family history of addiction disorders may predispose patients to hedonistic homoeostatic dysregulation.
Treatment
The evidence base for treatment of depression in Parkinson’s disease has been examined in a literature review and found to be limited (Reference Ghazi-Noori, Chung and DeaneGhazi-Noori et al, 2004). Tricyclic antidepressants have been shown to be effective in depression in Parkinson’s disease and may also improve motor symptoms by virtue of their anticholinergic activity (Reference Rascol, Goetz and KollerRascol et al, 2002). However, their side-effects, including cognitive impairment, may limit their use. Although SSRIs generally have fewer side-effects, the evidence that they work is not as strong, and some have extrapyramidal effects that exacerbate motor symptoms.
In psychosis, consideration should be given to decreasing or stopping antiparkinsonian drugs. Antipsychotic medications must be used with caution because of their extrapyramidal side-effects. There is good evidence that low-dose (<100 mg/day) clozapine can improve psychosis without worsening parkinsonism (Parkinson Study Group, 1999). Its use in patients with ischaemic heart disease, particularly elderly people, must be monitored carefully, as it can cause agranulocytosis and tachycardia. There is more limited evidence for the use of other atypicals, cholinesterase inhibitors (Reference Bergman and LernerBergman & Lerner, 2002) and odansetron to treat psychotic symptoms.
There is no current evidence supporting the use of cholinesterase inhibitors in dementia in Parkinson’s disease, although a randomised placebo-controlled trial has shown benefit with rivastigmine in Lewy body dementia (Reference McKeith, Del Ser and SpanoMcKeith et al, 2000).
Psychological treatments for depression in Parkinson’s disease have not been evaluated fully.
Huntington’s disease
Huntington’s disease is transmitted by a single autosomal dominant gene on the short arm of chromosome 4. The specific genetic defect has been identified as an expansion of the trinucleotide sequence CAG. In generations who inherit the disorder, the unstable sequence becomes longer, leading to the phenomenon of ‘anticipation’, whereby later generations manifest the disease at a younger age.
Prevalence of neuropsychiatric disorders
Estimates of the prevalence of psychiatric symptoms at the first presentation of Huntington’s disease are in the region of 30%. By and large, the neuropsychiatric presentations and their prevalence mirror those seen in Parkinson’s disease. In 3–6% of cases a schizophreniform psychosis is the first presentation of Huntington’s disease.
Clinical presentation
There has been significant interest in cognitive abnormalities in gene carriers identified by predictive testing prior to the full manifestation of the disease. The results have, unfortunately, been contradictory. Dispute remains as to whether there are cognitive differences between carriers and non-carriers, as well as to whether cognitive symptoms precede motor symptoms or vice versa (Reference Witjes-Ané, Vegter-van der Vlis and van VugtWitjes-Ané et al, 2003). Since George Huntington’s original description of the disorder, it has been well recognised that there is an increased rate of suicide in this group (Reference Schoenfeld, Myers and CupplesSchoenfeld et al, 1984).
Aetiology
Attempts (albeit with flawed methodologies) have been made to link the psychiatric signs and symptoms of Huntington’s disease to the length of the CAG repeat sequence, but no relationship has been found (Reference Weigell-Weber, Scmid and SpiegelWeigell-Weber et al, 1996).
Treatment
There are no controlled trials establishing the benefit of antidepressants for depression in Huntington’s disease. Atypical antipsychotics are preferable for psychoses, so as to avoid exacerbation of motor problems.
Other psychiatric disorders
Delirium
Clinical presentation
Delirium, often called an acute confusional state, is characterised by a disturbance of conscious level. The patient is obtunded (drowsy) or highly distractable. Attention and concentration are impaired (e.g. as demonstrated by poor performance on a digit span test). Mental state may fluctuate. The individual is neither alert nor oriented, and is likely to be agitated and frightened. Psychotic symptoms with hallucinations, often visual, and fleeting delusions may be elicited. Delirium may also present as a hypoactive withdrawn state akin to stupor.
Aetiology
A diagnosis of delirium always suggests an underlying physical or biological aetiology, and numerous physical problems, including drugs and drug withdrawal, may be aetiological factors.
Agitation is often present in delirium. If the patient has been treated with antipsychotics an important differential diagnosis of the agitation is akathisia. Poor sleep, pain, constipation and systemic illness may play a role.
Treatment
Management consists of making the patient safe and then seeking the underlying biological cause as well as treating the condition symptomatically. Nursing should take place in a well-lit side room with consistent staff. The environment should be calm and allow undisturbed sleep (Reference Inouye, Bogardus and CharpentierInouye et al, 1999).
Some patients will settle with reassurance and explanation. Relatives may be able to help. Haloperidol and lorazepam may be used to produce rapid sedation to manage agitation and aggression. The patient should be placed on regular nursing observations, with monitoring of respiration and neurological state. If sedation is required for more than 1 or 2 days, consider atypical antipsychotics such as olanzapine or quetiapine, which are less likely to produce extrapyramidal side-effects. If antipsychotics are not tolerated or cause intolerable side-effects, valproate, carbamazepine and beta-blockers may be helpful alternatives. Avoid drug combinations that may increase agitation and aggression by increasing confusion.
Sleep disorders
In this section, we briefly identify the sleep disorders whose presentations may initially be referred to the psychiatrist.
Narcolepsy
Prevalence
Narcolepsy is uncommon, with a prevalence of 0.025% in the general population.
Clinical presentations
Narcolepsy classically presents as excessive daytime sleepiness, with narcoleptic attacks, cataplexy (where the individual falls owing to sudden loss of muscle tone provoked by strong emotion, seen in 75% of patients), sleep paralysis (inability to move on waking or going to sleep, seen in 30%) and hypnagogic hallucinations (auditory, visual or tactile hallucinations on going to sleep). Only 10% of individuals present with all four phenomena. Alongside this tetrad, automatic behaviours have been reported in around one-third of patients. In these, despite appearing to be half-asleep, individuals engage in complex behaviours that are not recalled when they regain alertness.
Aetiology
Narcolepsy can be either familial or sporadic and a strong association with HLA haplotypes has been established. The HLA-DQB1*0602 marker is present in almost all individuals with narcolepsy and cataplexy, regardless of ethnicity. Hypocretins (orexins) are hypothalamic neuropeptides believed to play a role in regulating sleep and arousal. Abnormally low concentrations of hypocretin-1 (orexin-A) have been found in the cerebrospinal fluid of patients with narcolepsy. Research has also suggested a deficiency of hypocretin-2 in both narcolepsy and primary hypersomnia (Reference Ebrahim, Sharief and de LacyEbrahim et al, 2003).
Diagnosis and treatment
Investigation with overnight polysomnography often reveals sleep latency of less than 10 min and sleep-onset rapid eye movement (REM) periods. The multiple sleep latency test (Reference Carskadon, Dement and MitlerCarskadon et al, 1986), which confirms the presence or absence of REM activity as the patient begins to nap, is also helpful in supporting the diagnosis.
Management is focused on stopping or decreasing narcoleptic or cataplectic attacks. Stimulants such as methylphenidate or the newer modafinil have been shown to be effective.
REM sleep behaviour disorder
Clinical presentation
Individuals with REM sleep behaviour disorder act out their dreams, with limited awareness of their surroundings and often with violent or dangerous consequences that result in physical harm to themselves, property or others. The episodes arise during the middle to latter third of the night during REM sleep and are associated with loss of the normal atonia of REM sleep.
Aetiology
Rapid eye movement sleep behaviour disorder may occur idiopathically or together with disorders such as Parkinson’s disease, diffuse Lewy body disease, multiple system atrophy and Gullian–Barré syndrome. It has been reported that it can precede the diagnosis of a movement disorder by several years. As all episodes are associated with a loss of atonia, it is likely that the associated lesions are situated in the brainstem.
Treatment
Treatment with clonazepam has been shown to be effective, although making the sleeping environment safe is a sensible first-line measure.
Conclusions
The historical division that has existed between neurology and psychiatry is narrowing, much to the benefit of all patients with ‘brain disorders’. In this review we have sought to highlight some of the key clinical psychiatric presentations in neurological settings, as well as drawing attention to the associations between neurological illness and psychiatric syndromes. Often, treatment approaches (Box 4) are limited by poor-quality evidence and neuropharmacological complexity. Fortunately in the UK, neuropsychiatry is increasingly being recognised as a clinical sub-specialty, closely linked to general liaison psychiatry. If we are to continue to develop treatments for neuropsychiatric disorders and put them into practice then service developments, including closer liaison between neurology and psychiatry, will be necessary.
Box 4 Key points
-
• Effective practice demands clear communication between the neurologist and psychiatrist
-
• Always search for physical causes for symptoms, particularly in people with delirium and agitation, and consider effects of drugs, both prescribed and misused
-
• Start low and go slow when prescribing psychotropics, and avoid drug cocktails
-
• Do not leave depressive symptoms untreated; SSRIs are generally a safe first choice antidepressant
-
• When deciding on an antipsychotic for someone with brain injury or with Parkinson’s disease choose one with fewer extrapyramidal side-effects
-
• When prescribing psychotropics in epilepsy monitor anticonvulsant levels
Declaration of interest
None.
MCQs
-
1 In traumatic brain injury:
-
a the best predictor of outcome is the period of post-trauma amnesia
-
b late-onset agitation predicts long-term aggression
-
c aggression and agitation respond differently to medication
-
d suicidality has no association with hopelessness
-
e there is evidence of an association with schizophrenia.
-
-
2 Suicide rates have been shown to be higher in people with:
-
a Huntington’s disease
-
b hedonistic homoeostatic dysregulation
-
c traumatic brain injury
-
d REM sleep behaviour disorder
-
e stroke.
-
-
3 Research and investigations into sleep disorders have suggested that:
-
a hypocretins can be differentiated from orexins as having a role in sleep and arousal
-
b there is an HLA association with REM sleep behaviour disorder
-
c sleep latency of more than 10 min on overnight polysomnography is characteristic of narcolepsy
-
d clonazepam is the first-line treatment for narcolepsy
-
e REM sleep behaviour disorder can precede future movement disorders.
-
-
4 In Parkinson’s disease:
-
a psychosis is associated with decreases in antiparkinsonian medications
-
b clozapine is an appropriate treatment of psychotic symptoms
-
c hypersexuality, hypomania and pathological gambling can be seen with increased doses of dopamine-replacement medications
-
d dementia should be treated with rivastigmine as there is randomised controlled trial evidence in favour of this
-
e tricyclic antidepressants worsen motor symptoms.
-
-
5 People with epilepsy:
-
a should not be given psychotropic medications as they increase mean seizure frequency
-
b when treated with carbamazepine can be given fluoxetine without monitoring of serum levels
-
c can also have non-epileptic seizures
-
d as well as non-epileptic seizures need a lumbar puncture to ascertain prolactin levels for diagnostic purposes
-
e taking tricyclics may be more likely to experience a reduction in seizure threshold than those taking SSRIs.
-
MCQ answers
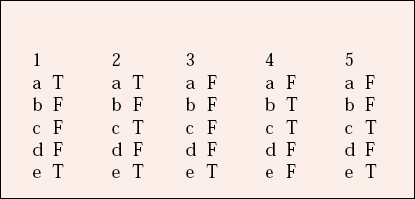
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | T | a | T | a | F | a | F | a | F |
| b | F | b | F | b | F | b | T | b | F |
| c | F | c | T | c | F | c | T | c | T |
| d | F | d | F | d | F | d | F | d | F |
| e | T | e | T | e | T | e | F | e | T |
Table 1 Psychiatric syndromes associated with stroke
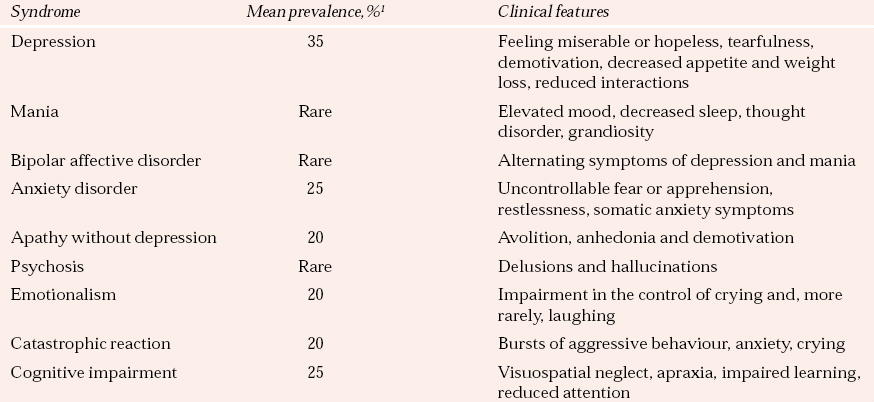
| Syndrome | Mean prevalence,% 1 | Clinical features |
|---|---|---|
| Depression | 35 | Feeling miserable or hopeless, tearfulness, demotivation, decreased appetite and weight loss, reduced interactions |
| Mania | Rare | Elevated mood, decreased sleep, thought disorder, grandiosity |
| Bipolar affective disorder | Rare | Alternating symptoms of depression and mania |
| Anxiety disorder | 25 | Uncontrollable fear or apprehension, restlessness, somatic anxiety symptoms |
| Apathy without depression | 20 | Avolition, anhedonia and demotivation |
| Psychosis | Rare | Delusions and hallucinations |
| Emotionalism | 20 | Impairment in the control of crying and, more rarely, laughing |
| Catastrophic reaction | 20 | Bursts of aggressive behaviour, anxiety, crying |
| Cognitive impairment | 25 | Visuospatial neglect, apraxia, impaired learning, reduced attention |
Table 2 Neuropsychiatric associations with epilepsy
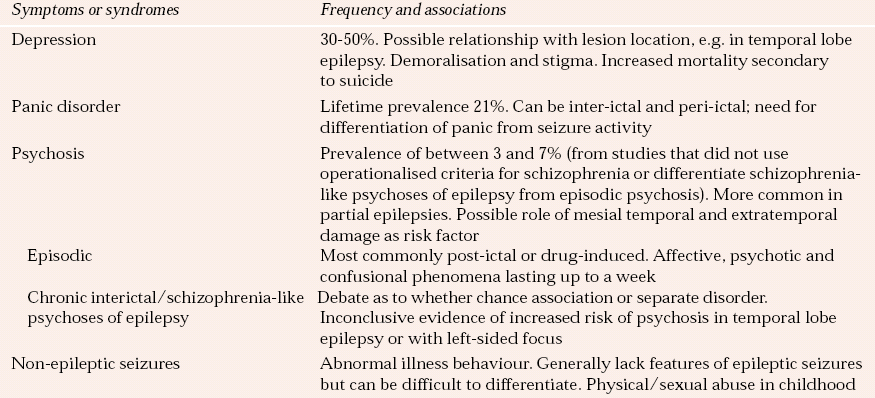
| Symptoms or syndromes | Frequency and associations |
|---|---|
| Depression | 30–50%. Possible relationship with lesion location, e.g. in temporal lobe epilepsy. Demoralisation and stigma. Increased mortality secondary to suicide |
| Panic disorder | Lifetime prevalence 21%. Can be inter-ictal and peri-ictal; need for differentiation of panic from seizure activity |
| Psychosis | Prevalence of between 3 and 7% (from studies that did not use operationalised criteria for schizophrenia or differentiate schizophrenia-like psychoses of epilepsy from episodic psychosis). More common in partial epilepsies. Possible role of mesial temporal and extratemporal damage as risk factor |
| Episodic | Most commonly post-ictal or drug-induced. Affective, psychotic and confusional phenomena lasting up to a week |
| Chronic interictal/schizophrenia-like psychoses of epilepsy | Debate as to whether chance association or separate disorder. Inconclusive evidence of increased risk of psychosis in temporal lobe epilepsy or with left-sided focus |
| Non-epileptic seizures | Abnormal illness behaviour. Generally lack features of epileptic seizures but can be difficult to differentiate. Physical/sexual abuse in childhood |
Table 3 Neuropsychiatric complications of Parkinson’s disease
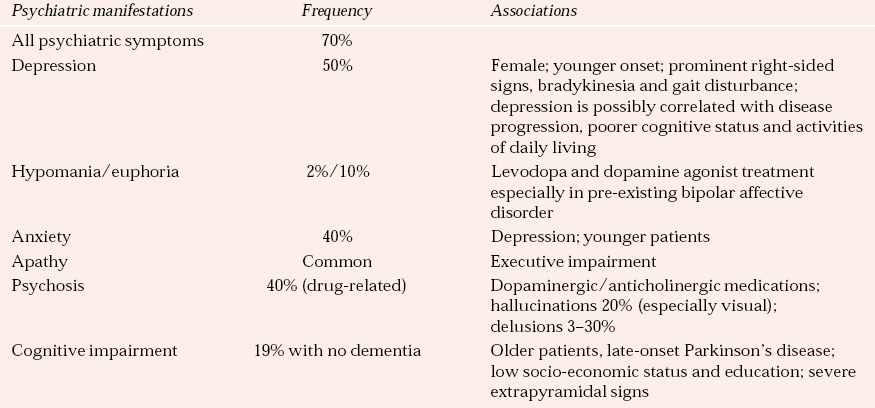
| Psychiatric manifestations | Frequency | Associations |
|---|---|---|
| All psychiatric symptoms | 70% | |
| Depression | 50% | Female; younger onset; prominent right-sided signs, bradykinesia and gait disturbance; depression is possibly correlated with disease progression, poorer cognitive status and activities of daily living |
| Hypomania/euphoria | 2%/10% | Levodopa and dopamine agonist treatment especially in pre-existing bipolar affective disorder |
| Anxiety | 40% | Depression; younger patients |
| Apathy | Common | Executive impairment |
| Psychosis | 40% (drug-related) | Dopaminergic/anticholinergic medications; hallucinations 20% (especially visual); delusions 3–30% |
| Cognitive impairment | 19% with no dementia | Older patients, late-onset Parkinson’s disease; low socio-economic status and education; severe extrapyramidal signs |








eLetters
No eLetters have been published for this article.