Delirium is an under-studied complex neuropsychiatric syndrome occurring in 11–42% of general medical in-patients (Reference Siddiqi, House and HolmesSiddiqi et al, 2006) and up to 50% of hospitalised elderly patients (Reference ColeCole, 2004). Historically, treatment has focused on underlying causes but the increasing attention to age-related conditions has heightened awareness that delirium, rather than being a benign transient state, is frequently persistent with an independent impact on functional capacity, morbidity and mortality (Reference Pitkälä, Laurila and StrandbergPitkälä et al, 2005).
The complex nature of delirium
Delirium is the accepted term for acute generalised disturbances of cognition, therefore superseding the many terms that relate to particular aetiologies or treatment settings (e.g. post-operative confusion, intensive care unit (ICU) psychosis, septic encephalopathy). It is a complex neuropsychiatric syndrome reflecting broad disturbance of brain function that includes a wide range of cognitive and non-cognitive features. Diagnosis is based on key contextual items (acute onset, fluctuating course, physical aetiology) along with global cognitive impairment (including orientation, memory, comprehension, executive function and visuospatial performance) with a disproportionate disturbance of attention. Disturbances of the sleep–wake cycle, perception, thought content, mood and affect also occur, with overt psychosis evident in 50% of those affected (Reference Meagher, Moran and RajuMeagher et al, 2007). Clinical subtypes defined by motor behaviour (hypoactive v. hyperactive v. mixed) differ with regard to detection (Reference Inouye, Foreman and MionInouye et al, 2001), treatment experience (Reference Meagher, O'Hanlon and O'MahonyMeagher et al, 1996; Reference Breitbart, Tremblay and GibsonBreitbart et al, 2002) and pathophysiology (Reference Balan, Leibovitz and ZilaBalan et al, 2003).
Delirium involves qualitative and quantitative alterations in consciousness, with diminished grasp of the immediate environment. Most ICU patients experience delirium as they emerge from coma, suggesting that disturbances of consciousness occupy a continuum. Delirium may present with non-specific prodromal features such as sleep disturbance, anxiety and calls for help, but cognitive impairment is often the first indication.
Patients with core symptoms of delirium not reaching full syndromal criteria (sub-syndromal delirium) experience outcomes similar to those for the full syndromal illness (Reference Marcantonio, Kiely and SimonMarcantonio et al, 2005). Furthermore, studies comparing diagnostic schema support broader definitions of delirium (Reference Laurila, Pitkala and StrandbergLaurila et al, 2004). This suggests that the present syndromal criteria may be too narrow and that the presence of any delirium symptoms warrants careful attention.
Aetiological attribution
Managing delirium requires a sound understanding of its causation. It is most common in hospitalised patients in whom there is a confluence of underlying predisposition (‘delirium readiness’) with acute precipitating insults. Age extremes, pre-existing cognitive impairment, severe comorbid illness and exposure to anticholinergic, benzodiazepine or opiate medications are especially robust predictors across populations (Reference MeagherMeagher, 2001). Single-aetiology delirium is the exception (Reference Meagher, Moran and RajuMeagher et al, 2007), with most cases involving the interaction of multiple factors, often sequentially, such that the rigorous and continuous reassessment of causation is a key component of management. Figure 1 compares the delirium profile of two patients with contrasting delirium predispositions exposed to a range of precipitating factors.
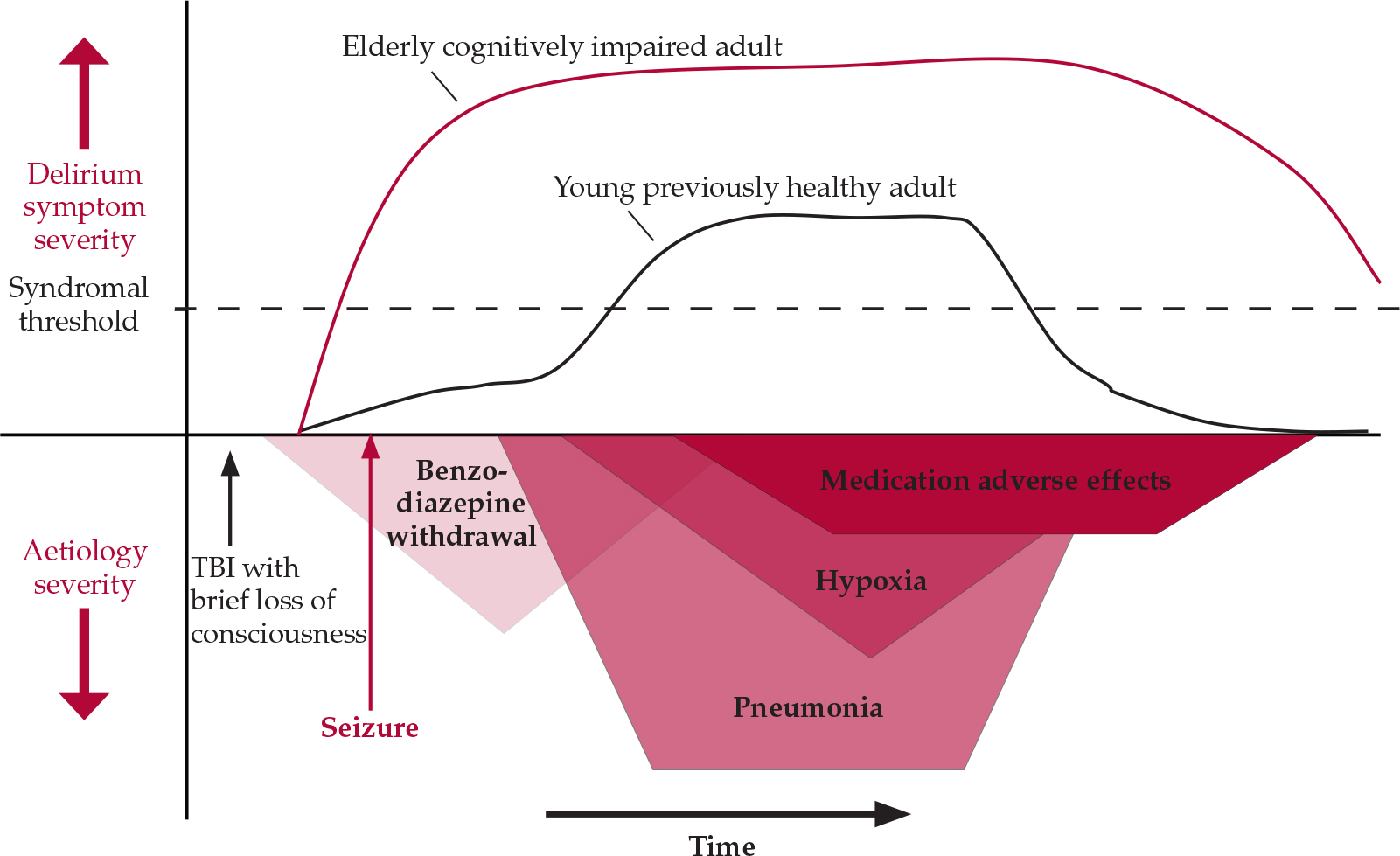
Fig. 1 The sequential causation of delirium: contrasting delirium profile in two patients with differing predispositions to delirium exposed to sequentially occurring deliriogenic insults after sustaining a head injury in a road traffic accident. TBI, traumatic brain injury.
Many risk factors for delirium can be modified by preventive interventions. Some operate as either protective or causative factors, depending on circumstances and magnitude of exposure. Benzodiazepines, for example, are a first-line treatment for alcohol withdrawal delirium but are also a recognised risk factor for delirium in ICU patients. Similarly, opioid toxicity can cause delirium but in hip-surgery patients delirium is nine times more frequent if their post-operative pain is undertreated (Reference Morrison, Magaziner and GilbertMorrison et al, 2003). Careful clinical judgement aligned to a willingness to discontinue potentially offending agents, at least for trial periods, can allow optimal exposure to deliriogenic drugs but even so, regular review with appropriate dose adjustments is required. Complementary medicines (e.g. henbane, Jimson weed, mandrake) are often perceived as benign despite their anticholinergic properties and are easily overlooked as a factor in delirium.
Detection
Episodes of delirium are often missed in clinical practice, especially in older patients and patients with hypoactive presentations or comorbid dementia (Reference Inouye, Foreman and MionInouye et al, 2001). Non-detection reflects the many misunderstandings about delirium (Box 1) and is associated with less than optimal care (e.g. premature hospital discharge) and poorer outcomes that include higher mortality (Reference Kakuma, du Fort and ArsenaultKakuma et al, 2003). A clear understanding of baseline cognition and personality is crucial to detection. Family and loved ones are uniquely equipped to detect the often subtle alterations in mental state (e.g. a sense of general malaise) that herald delirium, but their usefulness is often limited by a lack of awareness of the risk of delirium or of its various presentations even in high-risk populations. Individuals familiar with a patient's premorbid personality and functional level are less likely to ascribe subtle changes in mental state to being ‘cranky’ or ‘difficult’. Moreover, since about 50% of delirium is present on hospital admission (Reference Siddiqi, House and HolmesSiddiqi et al, 2006), meaning that baseline cognitive performance is not equivalent to the patient's normal function, collateral information is crucial to detection. In support, Reference Lundstrom, Edlund and KarlssonLundstrom et al (2005) found that interventions aimed at encouraging individualised care and enhancing interaction with caregivers significantly reduced the duration of delirium and mortality.
Box 1 Common misunderstandings about delirium
-
• The typical delirium presentation is of delirium tremens, i.e. agitated and floridly psychotic behaviour
-
• More severe delirium is associated with a greater degree of hyperactivity
-
• Quiet and well-behaved patients are generally cognitively intact
-
• Older people are normally forgetful and easily disoriented
-
• Irritability or vagueness generally reflects underlying personality rather than altered mental state
-
• Patients are easily offended or upset by simple tests of cognition
-
• The level of orientation and cognitive function is consistent over the 24-h cycle
-
• Delirium in those with advanced disease/cancer is rarely reversible
Nursing staff have contact with patients over the 24-h cycle and can readily liaise with visitors/family. However, without specific training nurses are not adept at recognising delirium because they tend to be overly reliant on orientation as a measure of cognition or mistake compliant behaviour as evidence of intact cognition (Reference Inouye, Foreman and MionInouye et al, 2001). Attitudes to ageing can cause nurses to normalise behaviour, for example explaining lethargy as ‘simply tired’ or tangentiality as ‘likes to tell stories’. Moreover, the fluctuating nature of delirium means that many patients have lucid periods (typically during morning ward rounds!). The accuracy of screening for delirium is greatly enhanced if efforts include formal cognitive assessment aligned to repeated, frequent (e.g. at least once per nursing shift) or continuous monitoring of mental state for delirium symptoms. Documentation of cognition as a fifth vital sign can increase the likelihood that daytime staff will respond to any difficulties noted during the previous night. Moreover, the high visibility of serial ratings of cognitive performance at the bedside can serve to highlight deteriorating mental state as it occurs.
Any alteration in cognition should trigger an assessment for possible delirium. The Mini-Mental State Examination (MMSE) is a commonly used screening test but it lacks sensitivity for delirium and emphasises orientation – an unreliable marker of delirium (Reference Meagher, Moran and RajuMeagher et al, 2007). The Confusion Assessment Method has been used in various settings, including the ICU and accident and emergency department, and can reliably detect delirium when used by trained clinicians but it lacks sensitivity when used by nurses. Sensitivity can be improved with versions adapted to de-emphasise acuteness of onset and fluctuating course (Reference Lemiengre, Nelis and JoostenLemiengre et al, 2006). Overall, simple repetitive cognitive assessment emphasising testing of attention, a relatively consistent feature of delirium, substantially increases detection (Box 2).
Box 2 Assessment for inattention and disorganised thinking
Attentiveness
Global attentiveness can be assessed during routine interaction: is there evidence of distractibility, perplexity, losing the thread of conversation, the need for cueing or rephrasing of questions, staring or generally lacking focus? Evidence of these features implies that more formal testing is indicated.
Informal structured assessments of attention:
-
• the ‘serial sevens’ subtraction task (it is usual to be able to negotiate at least five steps without error)
-
• spelling ‘world’ backwards
-
• listing the months of the year in reverse order (may be more appropriate if the patient's educational background is limited).
Formal assessment tests:
-
• The digit span task (Reference Hart, Best and SesslerHart et al, 1997) can be presented visually for patients with sensory difficulties. The ‘digit span backwards’ is more sensitive to cognitive impairment but subject to a bottoming-out effect; the ‘digit span forwards’ emphasises attention over working memory and is better at discriminating delirium. Usually 5–7 trials are conducted (one fewer for those over 65 years old). Scores ≤4 indicate significant attentional difficulties consistent with possible delirium (O'Keefe & Gosney, 1997).
Disorganised thinking
-
• Disorganised thinking is inevitably a subjective assessment based on evidence of rambling, irrelevant or incoherent speech with illogical flow of ideas or bizarre comments during conversation. Specific probes include asking about the patient's hobbies or areas of interest. Interpretation of proverbs (e.g. ‘one man's meat is another man's poison’) requires organised, rational, conceptual thinking.
Differential diagnosis
The principal disorders from which delirium must be distinguished are dementia and depression, especially with hypoactive presentations. Many depressive symptoms occur in delirium (e.g. disturbed vegetative functions) and with surprising frequency (e.g. thoughts of self-harm). Sustained disturbances of mood are more characteristic of mood disorders, whereas affective lability is more typical of delirium. In more complex cases it is useful to remember that patients with primary mood disorders rarely score significantly on formal measures of delirium severity (Reference Leonard, Spiller and KeenLeonard et al, 2008).
The traditional distinction between delirium and dementia according to acuteness of onset, fluctuating course and tendency for reversibility is less clear in those who experience the fluctuating symptom pattern of Lewy body dementia or who develop ‘persistent cognitive impairment’ following an episode of delirium. Delirium can be the harbinger of an underlying undiagnosed dementia, and persistent cognitive deficits may relate to medical problems that caused delirium, medication effects, consequences of inability to cooperate with treatments, or direct neurotoxicity of delirium. Where symptom onset and course do not distinguish their cause it is important to be aware that symptoms of delirium tend to dominate the clinical picture, and that delirium is characterised by greater disturbance of attention, more disorganised thinking and disorientation (Reference Trzepacz, Mulsant and DewTrzepacz et al, 1998).
Behavioural and psychological symptoms of dementia
Although clinicians know that superimposed delirium causes disturbed behaviour, the relationship between delirium and behavioural and psychological symptoms of dementia is under-studied despite the implications for investigation and treatment. Delirium is a medical emergency which may signal serious medical morbidity and as such it should be at the top of the diagnostic hierarchy. All patients with apparent behavioural and psychological symptoms of dementia should be investigated for delirium with careful history-taking and where necessary use of delirium assessment instruments (e.g. the Delirium Rating Scale–Revised–98; Reference Trzepacz, Mittal and TorresTrzepacz et al, 2001) that can reliably distinguish delirium from dementia.
Onset and course
Acute onset is less obvious in patients with pre-existing cognitive impairment, in whom worsening cognition is easily mistaken for the more gradual deterioration that occurs with dementia. Moreover, fluctuating course is a less reliable marker in patients with hypoactivity or dementia. Where baseline cognitive function is unclear or symptoms are not highly fluctuating the onus is to clarify that impairments are not due to delirium – it should not simply be presumed that they reflect pre-existing deficits.
Prevention
Primary prevention using multicomponent interventions for modifiable risk factors can reduce the frequency and severity of delirium in elderly medical and post-operative populations, with absolute risk reduction estimated at 13–19% (Reference ColeCole, 2004). Common elements include eliminating unnecessary medication, careful attention to hydration and nutrition, pain relief, correction of sensory deficits, sleep enhancement, early mobilisation and cognitive stimulation. Pharmacological prophylaxis in high-risk populations using haloperidol (Reference Kalisvaart, De Jonghe and BogaardsKalisvaart et al, 2005) and donepezil (Reference Sampson, Raven and NdhlovuSampson et al, 2006) can reduce delirium severity and duration but a better understanding of the magnitude of effect is needed before more routine use can be justified.
Healthcare costs are typically doubled in delirious patients (Reference Fick, Kolanowski and WallerFick et al, 2005) owing to greater complications and more prolonged hospitalisation. Reference Saravay, Kaplowitz and KurekSaravay et al (2004) studied the temporal evolution of episodes of delirium and found a close association between poor outcomes and the complications of uncontrolled symptoms. Close attention to the problems of hypostasis while providing a suitable environment to address hyperactivity and encourage cognitive recovery can reduce complications for many patients. Systematic detection and management protocols (an example of the latter appears later in this article) can improve the standard of care and reduce episode severity but must be regularly reinforced. Although follow-up studies have raised concerns regarding the benefits of such interventions beyond the period of hospitalisation, the value of short-term symptom control in allowing patients to participate in treatment decisions or simply optimising the quality of existence for terminally ill patients and their loved ones should not be underestimated.
Concepts from other areas of healthcare are increasingly being applied – using the model of infection control, delirium nurse specialists can identify risk factors, improve recognition and encourage standardised treatment. Others advocate the use of a non-medical ‘doula’ (as in obstetrics), who serves to address general aspects of care and broker more cohesive input from medical and nursing interactions (Reference Irving and ForemanIrving & Foreman, 2006).
Management of a delirium episode
The combined efforts of healthcare professionals and carers/family are needed to assess delirium carefully and provide an optimal environment for recovery. Information sheets (see Box 3 for sources) can raise awareness of the possibility and implications of delirium. A simple unambiguous care environment that restores a sense of control and promotes self-efficacy is the foundation of delirium management. Patients who have experienced an episode of delirium report that simple but firm communication, reality orientation, a visible clock and the presence of a relative contribute to a heightened sense of control. Light music-listening therapy (Reference McCaffrey and LocsinMcCaffrey & Locsin, 2004) can prevent understimulation while also buffering against noise extremes. Exposure to delirium risk factors and mortality can be reduced by moving the patient to a ‘delirium room’ – a small specialised unit devoted to comprehensive delirium-oriented treatment that minimises risk, aggravating factors and medication use with daily multidisciplinary reviews of progress (Reference Flaherty, Tariq and RaghavanFlaherty et al, 2003). As a general principle, cautious optimism regarding reversibility and prognosis is advocated.
Box 3 Further resources/information
-
• Let's Respect programme (www.olderpeoplesmentalhealth.csip.org.uk/lets-respect)
-
• The European Delirium Association: advocates for better services and fosters research activity across all disciplines. Various educational materials and a discussions forum are available (www.europeandeliriumassociation.com)
-
• The ICU Delirium and Cognitive Impairment Study Group: a US-based organisation fostering delirium care and research with critically ill patients. It offers teaching resources, including protocols for assessment and treatment (www.icudelirium.org)
-
• Mind: offers delirium-related resources, and a factsheet that includes information regarding symptoms and causes of cognitive disorders, including delirium (www.mind.org.uk)
-
• American Psychiatric Association: detailed delirium treatment guidelines, a quick reference guide and a patient and family guide (www.psych.org/psych_pract/treatg/quick_ref_guide/DeliriumQRG_4-15-05.pdf)
-
• For a detailed description of non-pharmacological measures for delirium management and an algorithm for managing severely disturbed patients see Reference MeagherMeagher (2001)
Drug treatment
Pharmacological management of delirium is currently based on empirical knowledge rather than well-designed efficacy studies. The fluctuating nature of delirium, spontaneous recovery and the impact of medical treatments make placebo-controlled studies essential to the evaluation of interventions, but studies are limited by the ethical constraints of treating a life-threatening condition with a placebo and by problems of consent. Nevertheless, there are now 20 published open-label treatment studies of antipsychotic agents, 10 using active comparators of which 4 were randomised (Reference Trzepacz, Meagher, Yudofsky and HalesTrzepacz & Meagher, 2007). More than two-thirds of delirious patients in these studies experienced rapid clinical improvement, typically after 2–6 days of treatment (Table 1). Moreover, a randomised controlled trial of haloperidol v. olanzapine v. non-drug treatment in elderly patients indicated that the response rates in the haloperidol (87.5%) and olanzapine (82%) treatment groups were similar and significantly greater than that in the non-drug treatment group (31%) (Reference Hua, Wei and HuiHua et al, 2006). Two placebo-controlled studies of haloperidol prophylaxis indicate less severe and shorter duration of delirium and reduced hospital stay in patients in the treatment arm even though antipsychotic treatment was used if delirium subsequently emerged in the placebo group, suggesting that earlier treatment is beneficial (Reference Kalisvaart, De Jonghe and BogaardsKalisvaart et al, 2005). Combined drug and non-pharmacological interventions (Reference Marcantonio, Flacker and WrightMarcantonio et al, 2001; Reference Pitkälä, Laurila and StrandbergPitkälä et al, 2005) achieve better results than non-pharmacological interventions alone (Reference Cole, McCusker and BellavanceCole et al, 2002).
Table 1 Prospective studies of pharmacological treatment in delirium1
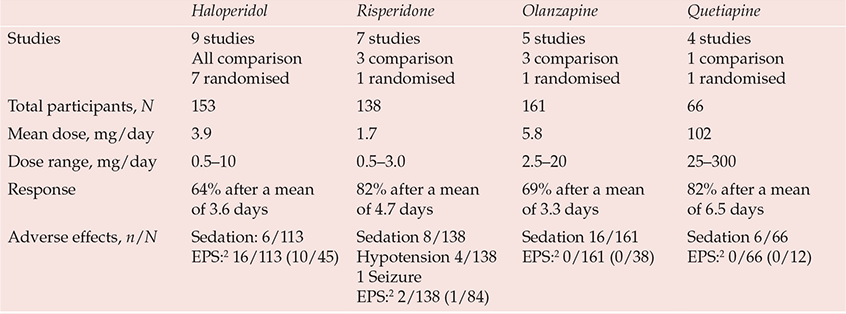
| Haloperidol | Risperidone | Olanzapine | Quetiapine | |
|---|---|---|---|---|
| Studies | 9
studies All comparison 7 randomised |
7
studies 3 comparison 1 randomised |
5
studies 3 comparison 1 randomised |
4
studies 1 comparison 1 randomised |
| Total participants, N | 153 | 138 | 161 | 66 |
| Mean dose, mg/day | 3.9 | 1.7 | 5.8 | 102 |
| Dose range, mg/day | 0.5–10 | 0.5–3.0 | 2.5–20 | 25–300 |
| Response | 64% after a mean of 3.6 days | 82% after a mean of 4.7 days | 69% after a mean of 3.3 days | 82% after a mean of 6.5 days |
| Adverse effects, n/N | Sedation:
6/113 EPS:2 16/113 (10/45) |
Sedation
8/138 Hypotension 4/138 1 Seizure EPS:2 2/138 (1/84) |
Sedation
16/161 EPS:2 0/161 (0/38) |
Sedation
6/66 EPS:2 0/66 (0/12) |
The lack of good-quality evidence is reflected in inconsistent treatment guidelines and wide variations in clinical practice. General physicians and geriatricians tend to reserve drug treatment as a last resort for disturbed behaviour (British Geriatrics Society & Royal College of Physicians, 2006; Reference InouyeInouye, 2006), whereas psychiatrists and physicians working in intensive care or palliative care use drug treatments more proactively, reflecting their greater familiarity with psychotropic agents (American Psychiatric Association, 1999; Reference MeagherMeagher, 2001; Reference Breitbart, Tremblay and GibsonBreitbart et al, 2002). Although drug-related causes are implicated in 30% of cases of delirium (Reference Gaudreau, Gagnon and HarelGaudreau et al, 2005) and delirium risk has been linked to most psychotropic agents and to polypharmacy, the need to rationalise medications should not be mistaken as a call always to reduce or discontinue them. Timely intervention, with careful dose titration and monitoring for adverse effects, can reduce both the degree and duration of delirium (Reference Lonergan, Britton and LuxenbergLonergan et al, 2007).
Underlying mechanisms of delirium
Most pharmacological strategies are based on the prevailing notion of a relative dopaminergic excess and cholinergic deficiency as the principal neurochemical aberration underlying delirium. Antipsychotics are more frequently prescribed for delirium involving psychosis or hyperactivity (Reference Meagher, O'Hanlon and O'MahonyMeagher et al, 1996) but response does not correspond to their inherent sedative potential (Reference Breitbart, Marotta and PlattBreitbart et al, 1996; Reference Lee, Won and LeeLee et al, 2005) and the timing and range of effect suggests that benefits are not closely related to antipsychotic action. Although studies exploring the impact of treatment on individual symptoms of delirium are lacking, response is generally defined as at least 50% reduction in total severity scores on the Delirium Rating Scale or the Revised Delirium Rating Scale that cannot be accounted for merely by effects on motor agitation or sleep disturbances. Younger patients with hyperactive presentations and patients without comorbid dementia respond better, but hypoactive patients also improve with antipsychotic treatment (Reference Platt, Breitbart and SmithPlatt et al, 1994; Reference Breitbart, Tremblay and GibsonBreitbart et al, 2002; Reference Liu, Juang and LiangLiu et al, 2004). Overall, existing studies suggest that delirium responds better to antipsychotics than do similar symptoms occurring in dementia (behavioural and psychological symptoms of dementia), perhaps reflecting the relatively greater disturbance of dopaminergic mechanisms in delirium (Reference Van der Cammen, Tiemeier and EngelhartVan der Cammen et al, 2006).
Haloperidol
Haloperidol remains the standard agent used to treat delirium because it has the most convincing evidence for benefit and is available in oral, intramuscular and intravenous preparations. Suggested doses are 1–2 mg every 4 h as needed, but with lower doses (e.g. 0.25–0.5 mg) for the elderly, very frail or populations with antipsychotic sensitivity (American Psychiatric Association, 1999). Uncontrolled agitated delirium can be life-threatening, especially in critically ill patients, and in such situations use of substantially higher doses has been reported without major adverse effects (Reference LevensonLevenson, 1995). However, higher doses (>4.5 mg/day) of haloperidol are associated with a greater incidence of side-effects (Reference Lonergan, Britton and LuxenbergLonergan et al, 2007). Algorithms guiding prescription at higher doses are available (e.g. Reference MeagherMeagher, 2001).
Atypicals and rivastigmine
Accumulating evidence supports the use of atypical antipsychotics (Table 1). Comparison studies suggest that olanzapine and risperidone have response rates similar to that of haloperidol but with reduced extrapyramidal side-effects (Reference Skrobik, Bergeron and DumontSkrobik et al, 2004). Agents (e.g. quetiapine) that are less prone to causing such side-effects are recommended where there is heightened susceptibility to extrapyramidal symptoms. Placebo-controlled studies support the use of low-dose clozapine for psychosis in Parkinson's disease and rivastigmine for psychosis in Lewy body dementia (Reference Leentjens and van der MastLeentjens & van der Mast, 2005). More sedating agents are preferable for highly agitated patients and, where possible, dose scheduling should facilitate a sleep–wake cycle recovery.
Benzodiazepines
Benzodiazepines are a first-line treatment for delirium related to substance use or seizures and they allow lower antipsychotic doses where extra sedation is desired. However, even in such cases delirium is often multi-aetiological and a concomitant antipsychotic may be required. Lorazepam can worsen mental state (Reference Breitbart, Marotta and PlattBreitbart et al, 1996) and is linked to risk of delirium in ICU patients (Reference Pandharipande, Shintani and PetersonPandharipande et al, 2006). Over 2 mg/day in lorazepam dose equivalents has been linked to a significantly elevated risk of delirium in patients with cancer (Reference Gaudreau, Gagnon and HarelGaudreau et al, 2005). Therapeutic aims should be explicit as benzodiazepine effects range from anxiolytic to sedative to hypnotic with ascending doses. Lorazepam is preferred because of its short-acting nature, absence of major active metabolites and relatively predictable bioavailability when given intramuscularly. Lower doses are required in elderly patients, those with respiratory or hepatic compromise, or receiving drugs that undergo extensive hepatic oxidative metabolism (e.g cimetidine, isoniazid). Effects can be rapidly reversed with flumazenil.
Procholinergics
Physostigmine can be used for delirium due to anticholinergic poisoning (Reference Burns, Linden and GraudinsBurns et al, 2000), but more routine use is limited by its propensity to cause gastrointestinal upset, seizures and cardiac arrhythmias. Procholinergic agents in treatment of more general delirium are supported by case reports, including otherwise treatment-resistant illness (Reference Kalisvaart, Boelaarts and De JongheKalisvaart et al, 2004).
Safety issues
Concerns about the potential risks to highly morbid patients pose an understandable barrier to pharmacological treatment of delirium. Where sedation is a particular concern (e.g. in hypoactive patients or those under mechanical ventilation) careful dose titration can be assisted by regular monitoring, if necessary using measures such as the Richmond Agitation and Sedation Scale (Reference Ely, Truman and ShintaniEly et al, 2003). Electrocardiogram monitoring is recommended with high-dose or intravenous haloperidol and where patients have a cardiac history or baseline QTc interval >450 ms.
Although haloperidol is a high-potency antipsychotic, extrapyramidal symptoms are uncommon in delirium treatment studies, including those using specific measurement instruments (Table 1). Extrapyramidal symptoms are even less frequent with atypical agents and have been reported in less than 1% of patients, contrasting with studies of behavioural and psychological symptoms of dementia, where rates of 10–15% are typical. This apparent low risk of extrapyramidal symptoms may reflect low dosing or the anticholinergic state that often underpins delirium. However, emerging akathisia can easily be mistaken for hyperactive delirium, and where doubt exists benzodiazepines allow reduced antipsychotic doses.
About 50% of delirium is superimposed on pre-existing dementia. Both pharmacological and non-pharmacological strategies are less effective in patients with concomitant dementia and the likelihood of somnolence and extrapyramidal symptoms is elevated. Additional concerns regarding cerebrovascular incidents also militate against antipsychotic use, but the relative risks of short-term use in delirium are considerably less than those associated with more prolonged use in the treatment of behavioural and psychological disturbances in dementia. Overall, the rationale in patients with concomitant dementia is less clear. At the very least lower doses should be used, with careful monitoring for adverse effects. More prolonged use should not occur without clear benefits.
Figure 2 shows a treatment algorithm for delirium highlighting the principal considerations in choice of drug and dose.
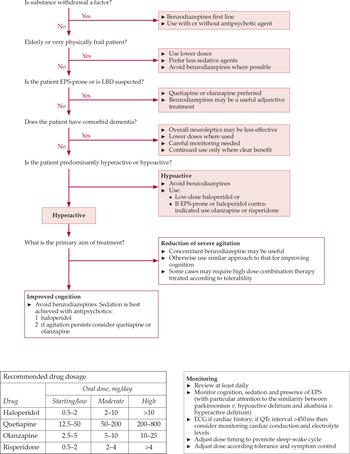
Fig. 2 Delirium treatment algorithm and guidance regarding dosage and monitoring (reproduced with permission of Milford Hospice Palliative Care Centre). ECG, electrocardiogram; EPS, extrapyramidal symptoms; LBD, Lewy body dementia.
Post-episode/post-discharge care
Pharmacological treatment of delirium should continue until symptoms have fully resolved. It is the consensus that discontinuation should be attempted after 1 week symptom-free (Reference Alexopoulos, Streim and CarpenterAlexopoulos et al, 2004). In reality many patients are discharged before resolution of symptoms, resulting in elevated subsequent mortality rates. The psychological aftermath of delirium is under-studied but around 50% of patients can recall the episode and many have distressing recollections 6 months later (Reference O'KeeffeO'Keeffe, 2005). Delirium is also distressing for carers and families and can contribute to abnormal bereavement reactions. A follow-up visit with patients and, if possible, their carers, can clarify the meaning of delirium, its difference from dementia and provide an opportunity to address future risk factors and medication adjustments.
Conclusions
Although delirium remains under-studied there is gathering information that can inform therapeutic efforts. Primary prevention by attending to modifiable risk factors can reduce delirium risk. Earlier recognition requires an appreciation of the complex differential diagnosis and multifactorial aetiology of delirious states combined with routine and systematic screening for altered mental states and cognitive impairment. The combination of non-drug strategies with judicious use of pharmacological treatments can shorten episode duration and reduce the likelihood of complications that contribute to prolonged hospitalisation and adverse outcomes. Treatment efforts should continue after hospital discharge by addressing ongoing rehabilitation needs and reducing future risk factors.
Declaration of interest
D.M. and M.L. are in receipt of an unrestricted educational grant from Astra Zeneca Pharmaceuticals. D.M. has acted as an advisory consultant for Pfizer, Eli-Lilly, Bristol-Meyers and Janssen Pharmaceuticals and has received travel support from Smith-Kline Beecham, Eli-Lilly, Astra-Zeneca, Novartis, Wyeth, and Bristol-Meyers Pharmaceuticals Ltd. M.L. is the joint winner of the Lundbeck Neuroscience Bursary, 2008.
MCQs
-
1 When screening for cognitive disturbance in patients at risk for delirium:
-
a the MMSE is the preferred instrument
-
b disorientation is a reliable marker of its presence
-
c the Confusion Assessment Method is a sensitive measure when used by nurses
-
d the digit span is simple but effective
-
e simple tests should be avoided because they may offend elderly patients.
-
-
2 Regarding causes of delirium:
-
a benzodiazepines are typically protective in action
-
b most cases of delirium can be linked to a single causative factor
-
c delirium aetiology should be reassessed regularly during an episode
-
d type of surgery is not relevant to delirium risk
-
e predisposing factors are less important than precipitating factors.
-
-
3 In delirium prevention:
-
a most cases can be prevented with careful attention to delirium risk factors
-
b prophylactic use of haloperidol has been demonstrated to reduce delirium incidence
-
c combined drug and non-pharmacological approaches are superior in delirium prevention
-
d the ‘delirium room’ is an experimental paradigm used to study delirium causation
-
e patients with pre-existing cognitive impairments benefit most from preventive interventions.
-
-
4 The use of antipsychotic agents in delirium
-
a is supported by placebo-controlled efficacy studies
-
b is indicated for patients with psychotic features only
-
c is frequently associated with the emergence of extrapyramidal symptoms
-
d is contraindicated in patients with hypoactivity
-
e is associated with clinical improvement in two-thirds of patients within 1 week.
-
-
5 In the longer-term management of delirium
-
a antipsychotic agents should be continued for 3 months
-
b most patients have no recall of the episode
-
c most patients openly discuss their experiences
-
d careful consideration of risk factors can prevent further episodes
-
e procholinergic agents are the treatment of choice.
-
MCQ answers

| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | F | a | F | a | F | a | F | a | F |
| b | F | b | F | b | F | b | F | b | F |
| c | F | c | T | c | T | c | F | c | F |
| d | T | d | F | d | F | d | F | d | T |
| e | F | e | F | e | F | e | T | e | F |
Acknowledgements
We acknowledge the support and cooperation of the nursing and medical staff at Milford Hospice Palliative Care Centre who participated in the formulation of the treatment algorithm contained herein. We also thank Professor Paula Trzepacz for her helpful comments and suggestions on an earlier version of this article.






eLetters
No eLetters have been published for this article.