Book contents
- Decriminalizing Mental Illness
- Decriminalizing Mental Illness
- Copyright page
- Contents
- Contributors
- Part I Introduction/Description of the Problem
- Part II Solutions
- Part III Psychopharmacological Treatment Considerations
- Part IV Nonpsychopharmacological Treatment Considerations
- Part V Criminal Justice and Social Considerations
- Index
- References
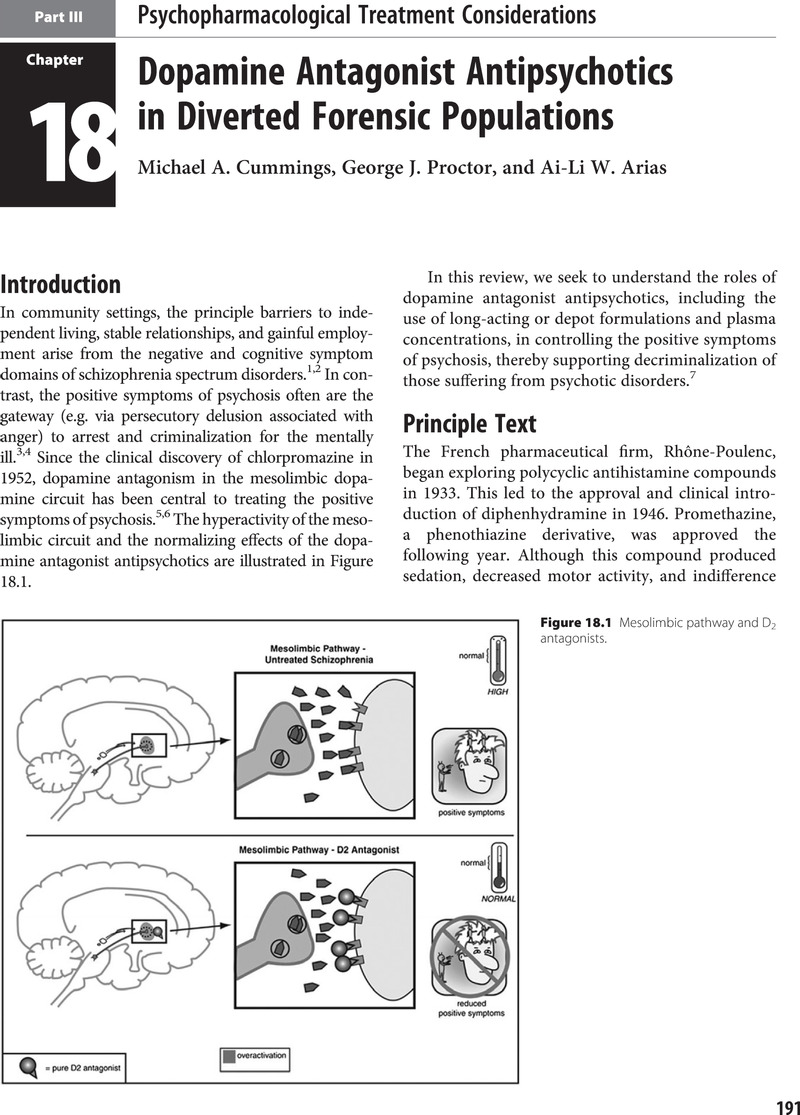
Part III - Psychopharmacological Treatment Considerations
Published online by Cambridge University Press: 19 October 2021
- Decriminalizing Mental Illness
- Decriminalizing Mental Illness
- Copyright page
- Contents
- Contributors
- Part I Introduction/Description of the Problem
- Part II Solutions
- Part III Psychopharmacological Treatment Considerations
- Part IV Nonpsychopharmacological Treatment Considerations
- Part V Criminal Justice and Social Considerations
- Index
- References
Summary
- Type
- Chapter
- Information
- Decriminalizing Mental Illness , pp. 191 - 220Publisher: Cambridge University PressPrint publication year: 2021



